All Photos(1)
About This Item
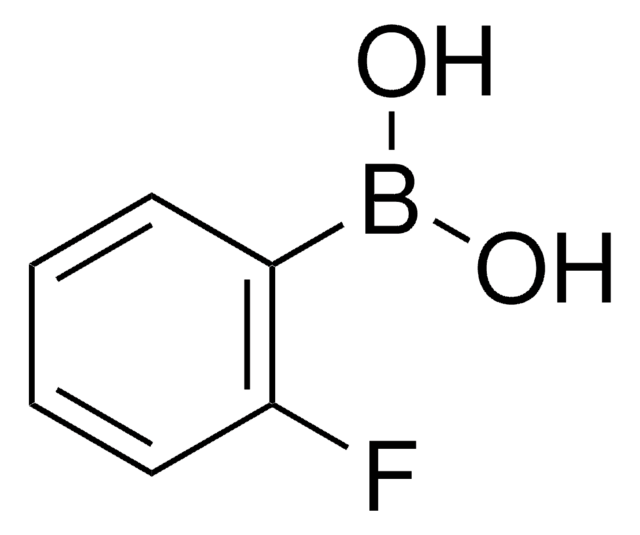
Linear Formula:
F2C6H3B(OH)2
CAS Number:
Molecular Weight:
157.91
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
147-149 °C (lit.)
SMILES string
OB(O)c1c(F)cccc1F
InChI
1S/C6H5BF2O2/c8-4-2-1-3-5(9)6(4)7(10)11/h1-3,10-11H
InChI key
DBZAICSEFBVFHL-UHFFFAOYSA-N
Related Categories
Application
2,6-Difluorophenylboronic acid can be used:
- As a substrate in the model reaction of Suzuki–Miyaura coupling with 4-chloro-3-methylanisole.
- To prepare 4-bromo-2,3′,5′,6-tetrafluorobiphenyl, a key intermediate for the synthesis of 2,6-difluorinated oligophenyls applicable in organic semiconductors.
- To prepare ethyl 4-(2,6-difluorophenyl)nicotinate, a key intermediate for the synthesis of 4-phenyl pyridine based potent TGR5 agonists.
Other Notes
Contains varying amounts of the anhydride
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enhancing charge mobilities in selectively fluorinated oligophenyl organic semiconductors: a design approach based on experimental and computational perspectives
Maiti B, et al.
Journal of Material Chemistry C, 7(13), 3881-3888 (2019)
Design and preparation of new palladium precatalysts for C-C and C-N cross-coupling reactions
Bruno NC, et al.
Chemical Science, 4(3), 916-920 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service