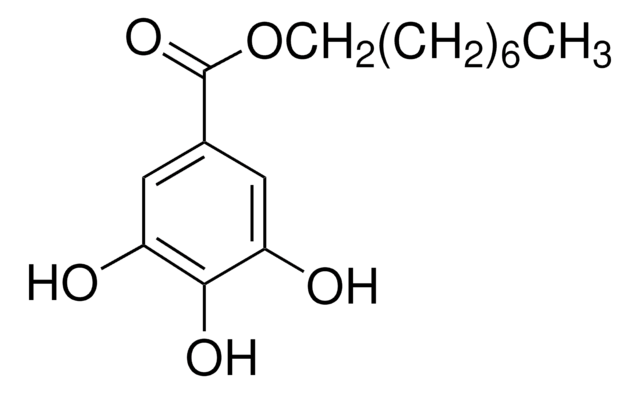
48660
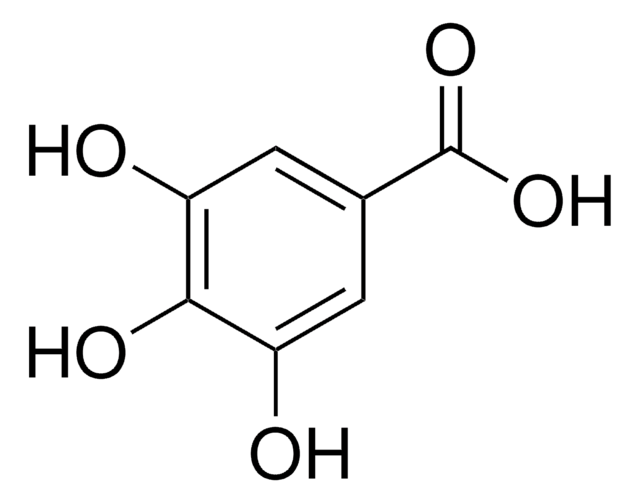
Lauryl gallate
antioxidant, ≥99.0% (HPLC)
Synonym(s):
Dodecyl gallate
About This Item
Recommended Products
Quality Level
assay
≥99.0% (HPLC)
form
solid
mp
94-97 °C (lit.)
SMILES string
CCCCCCCCCCCCOC(=O)c1cc(O)c(O)c(O)c1
InChI
1S/C19H30O5/c1-2-3-4-5-6-7-8-9-10-11-12-24-19(23)15-13-16(20)18(22)17(21)14-15/h13-14,20-22H,2-12H2,1H3
InChI key
RPWFJAMTCNSJKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Properties of artificial phospholipid membranes containing lauryl gallate or cholesterol: This study explores how lauryl gallate affects membrane structure, impacting its biological functions (Jurak et al., 2018).
- Potential of Lauryl Gallate as Stability and Recyclability Improver of Poly (Butylene succinate-co-adipate): Lauryl gallate enhances the stability and recyclability of polyesters, important for sustainable materials (Rossi et al., 2024).
- Lauryl Gallate Activity and Streptococcus mutans: The study evaluates the antibacterial effects of lauryl gallate against Streptococcus mutans, showing significant biofilm reduction and gene expression impact (Gabe et al., 2020).
- Dodecyl gallate as a pro-ecological antioxidant for food packing materials: This research investigates the use of lauryl gallate as a green antioxidant, enhancing the age-resistance of food packaging (Masek et al., 2014).
signalword
Warning
hcodes
Hazard Classifications
Skin Sens. 1
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service