525057
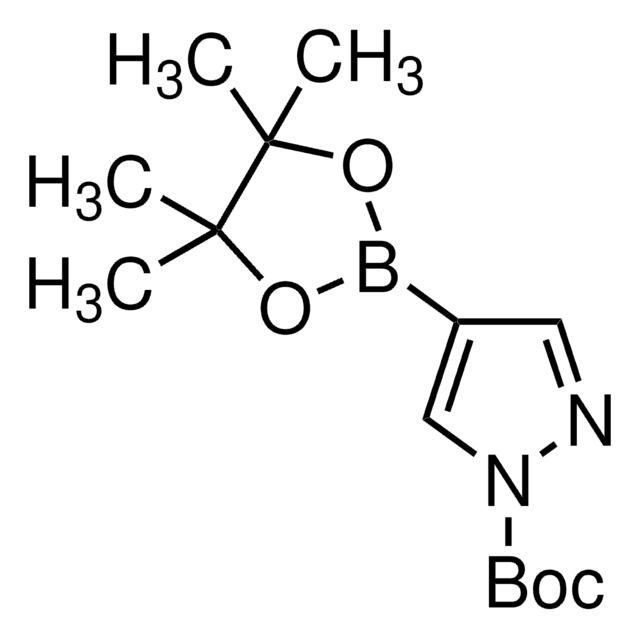
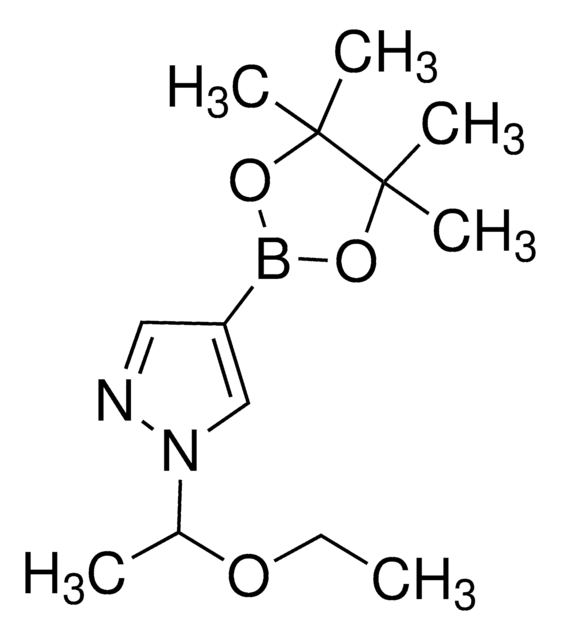
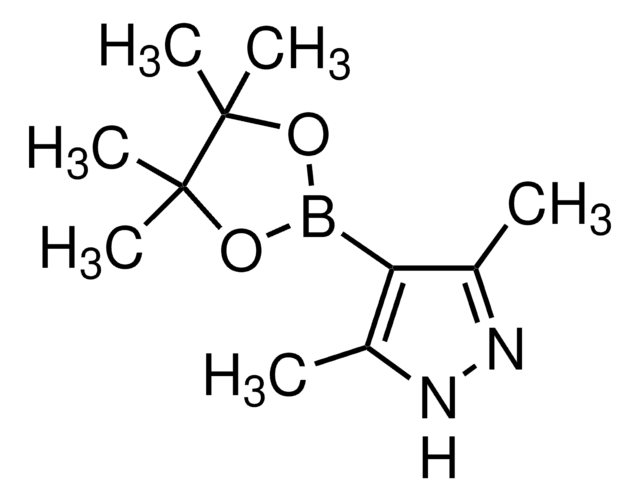
4-Pyrazoleboronic acid pinacol ester
97%
Synonym(s):
4,4,5,5-Tetramethyl-2-(1H-pyrazol-2-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(1H-pyrazol-4-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(pyrazol-4-yl)-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole, Pyrazol-4-ylboronic acid pinacol ester
About This Item
Recommended Products
Quality Level
assay
97%
form
solid
mp
142-146 °C (lit.)
SMILES string
CC1(C)OB(OC1(C)C)c2cn[nH]c2
InChI
1S/C9H15BN2O2/c1-8(2)9(3,4)14-10(13-8)7-5-11-12-6-7/h5-6H,1-4H3,(H,11,12)
InChI key
TVOJIBGZFYMWDT-UHFFFAOYSA-N
Related Categories
Application
- Suzuki-Miyaura cross-couplings
- Ruthenium-catalyzed asymmetric hydrogenation
Reagent used in preparation of inhibitors of many highly significant therapeutic enzymes and kinases containing the privileged scaffold pyrazole, including
- VEGF
- Aurora
- Rho (ROCK)
- Janus Kinase 2 (JAK)
- c-MET
- ALK
- S-nitrosoglutathione reductase
- CDC7
- Acetyl-CoA carboxylase
- Prosurvival Bcl-2 protein
- Viral RNA-Dependent RNA polymerase
- Long Chain Fatty Acid Elongase 6
- PI3
- AKT
- Chk1
- Protein Kinase B
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service