All Photos(1)
About This Item
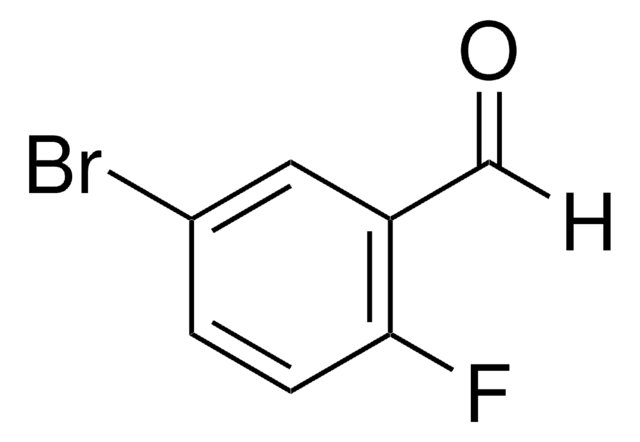
Linear Formula:
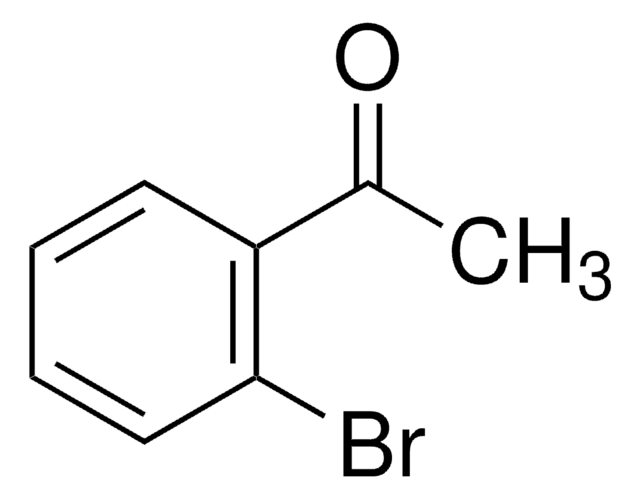
BrC6H4(F)CHO
CAS Number:
Molecular Weight:
203.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
mp
51-56 °C (lit.)
storage temp.
2-8°C
SMILES string
Fc1ccc(Br)c(C=O)c1
InChI
1S/C7H4BrFO/c8-7-2-1-6(9)3-5(7)4-10/h1-4H
InChI key
CJUCIKJLMFVWIS-UHFFFAOYSA-N
General description
2-Bromo-5-fluorobenzaldehyde can be prepared by reacting 2-bromo-5-fluorotoluene with N-bromosuccinimide. Its crystals exhibit monoclinic crystal system and space group P21/c.
Application
2-Bromo-5-fluorobenzaldehyde may be used in the synthesis of dihydrobenzoxaboroles bearing aryl, heteroaryl or vinyl substituents at the 1-position and 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole.
wgk_germany
WGK 3
flash_point_f
No data available
flash_point_c
No data available
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stephen J Baker et al.
Journal of medicinal chemistry, 49(15), 4447-4450 (2006-07-21)
A structure-activity relationship investigation for a more efficacious therapy to treat onychomycosis, a fungal infection of the toe and fingernails, led to the discovery of a boron-containing small molecule, 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), which is currently in clinical trials for onychomycosis topical
2-Bromo-5-fluorobenzaldehyde.
Tureski RE and Tanski JM.
Acta Crystallographica Section E, Structure Reports Online, 69(8), o1246-o1246 (2013)
Nuclear spin-spin coupling via nonbonded interactions. 4. Fluorine-fluorine and hydrogen-fluorine coupling in substituted benzo [c] phenanthrenes.
Mallory FB, et al.
The Journal of Organic Chemistry, 50(4), 457-461 (1985)
Izabela D Madura et al.
Acta crystallographica. Section E, Structure reports online, 67(Pt 2), o414-o415 (2011-04-28)
In the crystal structure of the title compound, C(7)H(6)BFO(2), a broad-spectrum anti-fungal drug (AN2690), the planar [maximum deviation 0.035 (1) Å] mol-ecules form centrosymmetric R(2) (2)(8) dimers via strong O-H⋯O hydrogen bonds. The dimers are arranged into layers by weak inter-molecular C-H⋯O
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service