All Photos(1)
About This Item
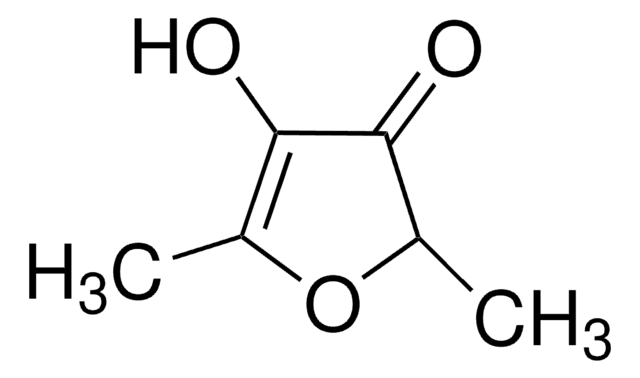
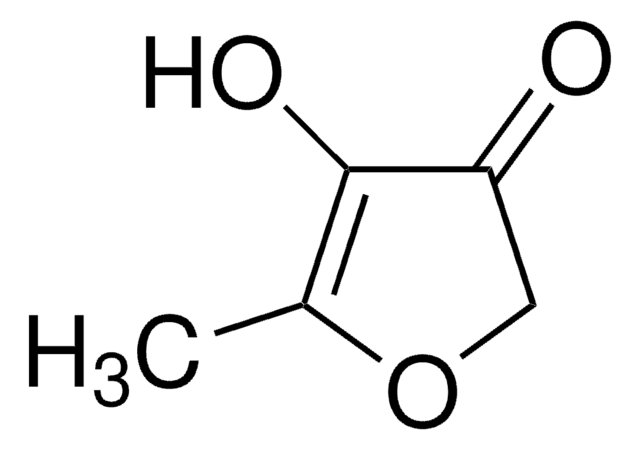
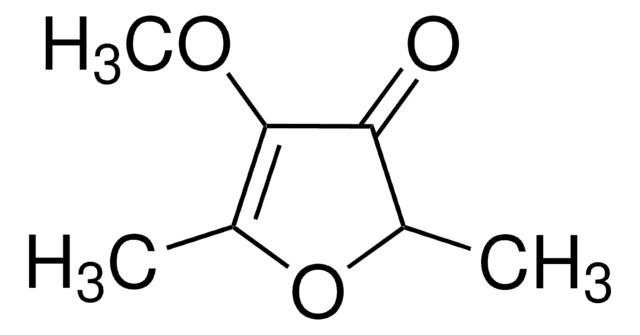
Linear Formula:
C4OH2OCH3OH
CAS Number:
Molecular Weight:
114.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
129-133 °C (lit.)
SMILES string
CC1=C(O)C(=O)CO1
InChI
1S/C5H6O3/c1-3-5(7)4(6)2-8-3/h7H,2H2,1H3
InChI key
DLVYTANECMRFGX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Hydroxy-5-methyl-3-furanone may be used in oxidoreductase assay for the evaluation of oxidoreductase activity.
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Highly effective inhibition of biofilm formation by the first metagenome-derived AI-2 quenching enzyme.
Weiland-Brauer N, et al.
Frontiers in Microbiology, 7 (2016)
Reinvestigation of the reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone.
A Ravagli et al.
Journal of agricultural and food chemistry, 47(12), 4962-4969 (1999-12-22)
The reaction between 2-furancarboxaldehyde and 4-hydroxy-5-methyl-3(2H)-furanone was reinvestigated as a part of a systematic study on low molecular weight colored compounds from the Maillard reaction. In acetic acid/piperidine, besides 2-(2-furanylmethylene)-4-hydroxy-5-methyl-3(2H)-furanone (1) and 5-[2-(2-furanyl)ethenyl]-2-(2-furanylmethylene)-4-hydroxy-5-methyl -3( 2H)-furanone (2), four novel compounds, 15a
Tobias Hauck et al.
Journal of agricultural and food chemistry, 51(5), 1410-1414 (2003-02-20)
Formation of the flavor compound and precursor 4-hydroxy-5-methyl-3[2H]-furanone (HMF, norfuraneol) was demonstrated in cytosolic protein extracts obtained from Zygosaccharomyces rouxii after incubation with a number of carbohydrate phosphates. 4-Hydroxy-5-methyl-3[2H]-furanone was produced from d-fructose-1,6-diphosphate, d-fructose-6-phosphate, d-glucose-6-phosphate, 6-phosphogluconate, d-ribose-5-phosphate, and d-ribulose-1,5-diphosphate. Enzyme
F B Whitfield et al.
Journal of agricultural and food chemistry, 47(4), 1626-1634 (1999-11-24)
Reaction of 4-hydroxy-5-methyl-3(2H)-furanone (HMF) with cysteine or hydrogen sulfide at pH 4.5 for 60 min at 140 degrees C produced complex mixtures of volatile compounds, the majority of which contained sulfur. Sixty-nine compounds were identified, some tentatively, by GC/MS. These
H J Kim et al.
Advances in experimental medicine and biology, 434, 91-99 (1998-05-23)
The chemometric principle was used to derive a guideline for obtaining a simple "yes or no" answer about the sterility of food particulates heated at aseptic processing temperatures. A quadratic temperature pulse model was used to estimate bacterial destruction from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service