591688
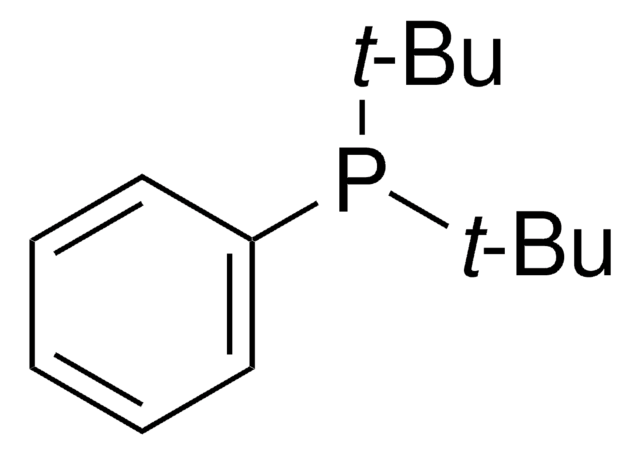
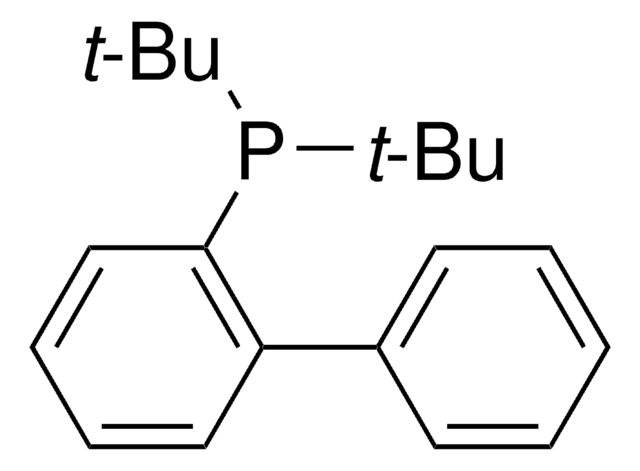
tert-Butyldiphenylphosphine
97%
Synonym(s):
NSC 244302
About This Item
Recommended Products
Quality Level
assay
97%
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Cross Couplings
bp
144-146 °C/2 mmHg (lit.)
mp
52-57 °C (lit.)
functional group
phosphine
SMILES string
CC(C)(C)P(c1ccccc1)c2ccccc2
InChI
1S/C16H19P/c1-16(2,3)17(14-10-6-4-7-11-14)15-12-8-5-9-13-15/h4-13H,1-3H3
InChI key
QZUPHAGRBBOLTB-UHFFFAOYSA-N
Related Categories
Application
- Nickel/Lewis acid-catalyzed carbocyanation of alkynes with acetonitrile or substituted acetonitriles
- Palladium catalyzed hydrocarboxylation of acetylene with carbon monoxide to acrylic acid under mild conditions
- Palladium to form a catalyst for methoxycarbonylation reactions
- Ru-catalyzed transfer hydrogenation in reductive coupling of disubstituted allenes with aldehydes
- Stereoselective silylation by dehydrogenative Si-O coupling with Si-stereogenic silanes
- Phosphine ligand in nickel-catalyzed carbocyanation of alkynes
- Monodentate P-Donor Ligands (LKB-P)
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service