597910
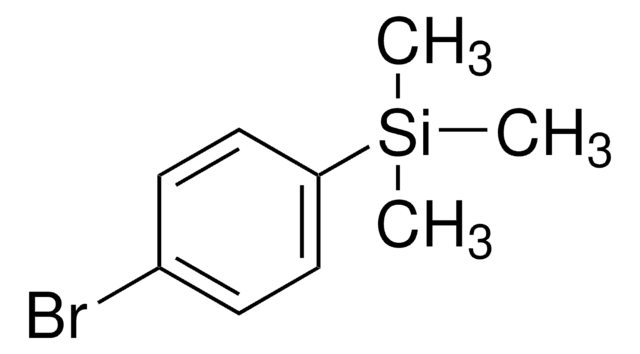
(4-Chlorophenyl)triethoxysilane
97%
Synonym(s):
1-Chloro-4-triethoxysilylbenzene
About This Item
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.474 (lit.)
bp
82-84 °C/0.07 mmHg (lit.)
density
1.069 g/mL at 25 °C (lit.)
SMILES string
CCO[Si](OCC)(OCC)c1ccc(Cl)cc1
InChI
1S/C12H19ClO3Si/c1-4-14-17(15-5-2,16-6-3)12-9-7-11(13)8-10-12/h7-10H,4-6H2,1-3H3
InChI key
AFILDYMJSTXBAR-UHFFFAOYSA-N
Related Categories
Application
- To facilitate the connection between dipolar mixed monolayers and zinc oxide in photovoltaic devices.
- As a substrate in the Ni-catalyzed decarboxylative coupling with alkynyl carboxylic acids to yield substituted diarylalkynes.
- As a substrate in the Hiyama cross-coupling reactions with 3-iodoazetidines to yield substituted 3-arylazetidines.
- As an intermediate in the synthesis of tripod-shaped oligo(p-phenylene)s, which are used in the surface immobilization of gold and CdS quantum dots for sensor applications.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
> 110 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
A viable alternative to the popular Stille and Suzuki coupling reactions mainly due to the formation of nontoxic byproducts and stability to many reaction conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service