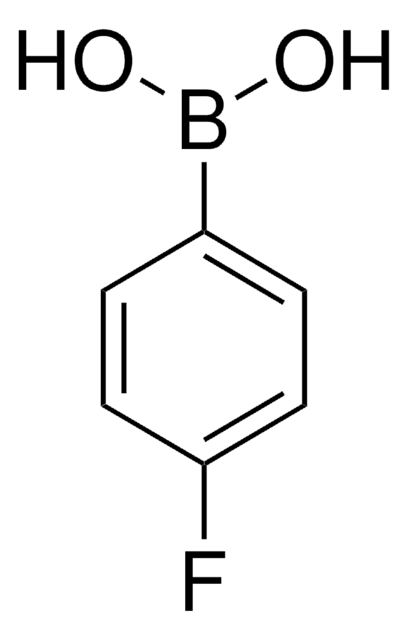
673854
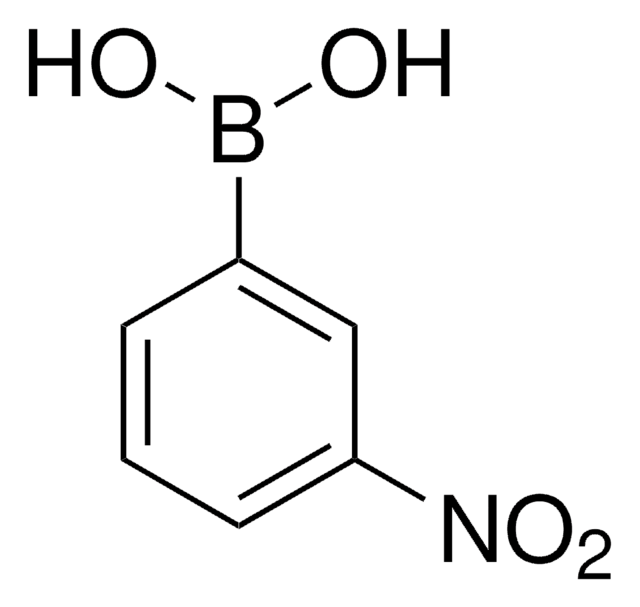
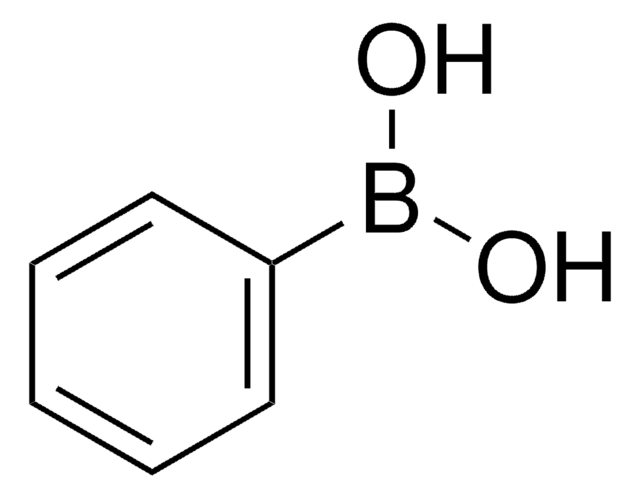
4-Nitrophenylboronic acid
≥95.0%
Synonym(s):
4-Nitrobenzeneboronic acid, p-Nitrophenylboronic acid, p-nitro-benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(O2N)C6H4(B(OH)2)
CAS Number:
Molecular Weight:
166.93
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥95.0%
form
solid
mp
285-290 °C (dec.)
SMILES string
OB(O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H6BNO4/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,9-10H
InChI key
NSFJAFZHYOAMHL-UHFFFAOYSA-N
Related Categories
Application
Reagent used for
Reagent used in Preparation of
- Ligand-free palladium-catalyzed Suzuki-Miyaura cross-couplings
- Ruthenium catalyzed direct arylation of benzylic sp3 carbons of acyclic amines
- Diels-Alder or C-H activation reactions
- Regioselective Suzuki-Miyaura coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulations
- N-arylation of phenylurea using copper acetylacetonate catalyst
- Environmentally benign one-pot synthesis through a double arylation process
- Copper-mediated cyanations
- copper-catalyzed arylations
- Regioselective glycosylations
- Suzuki couplings followed by arylations
- X-ray absorption on rhodium-grafted hydrotalcite catalyst for heterogeneous 1,4-addition reaction of organoboron reagents to electron deficient olefins
Reagent used in Preparation of
- Combretastatin analogs as potential antitumor agents
- Human immunodeficiency virus (HIV) protease inhibitors with antiviral activities against drug-resistant viruses
Other Notes
May contain varying amounts of anhydride
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rhodium-grafted hydrotalcite catalyst for heterogeneous 1,4-addition reaction of organoboron reagents to electron deficient olefins
Motokura, K.; et al.
Green Chemistry, 13, 2416-2422 (2011)
An efficient copper-catalyzed one-pot synthesis of diaryl thioethers by coupling of arylboronic acids with potassium ethyl xanthogenate under mild conditions
Wang, L.; et al.
Synlett, 20, 3041-3045 (2011)
An efficient access to 2,3-diarylimidazo[1,2-a]pyridines via imidazo[1,2-a]pyridin-2-yl triflate through a Suzuki cross-coupling reaction-direct arylation sequence
Marhadour, S.; et al.
Tetrahedron Letters, 53, 297-300 (2012)
Abdallah Hamze et al.
ChemMedChem, 6(12), 2179-2191 (2011-10-13)
A novel class of isocombretastatin A-4 (isoCA-4) analogues with modifications at the 3'-position of the B-ring by replacement with C-linked substituents was studied. Exploration of the structure-activity relationships of theses analogues led to the identification of several compounds that exhibit
Tetrahedron, 63, 6131-6131 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service