75056
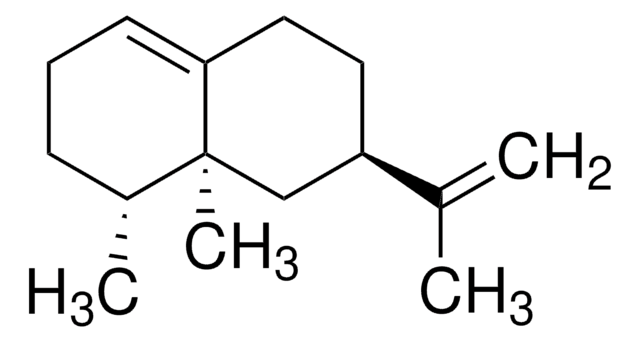
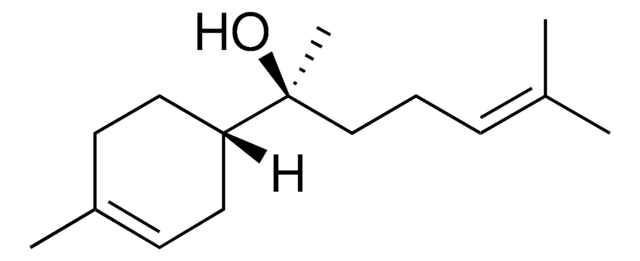
(+)-Valencene
technical, ≥70%
Synonym(s):
(3R,4aS,5R)-4a,5-Dimethyl-3-isopropenyl-1,2,3,4,4a,5,6,7-octahydronaphthalene
About This Item
Recommended Products
grade
technical
Quality Level
assay
≥70%
form
liquid
optical activity
[α]20/D +100±25°, neat
refractive index
n20/D 1.504 (lit.)
bp
274 °C (lit.)
density
0.92 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[C@@H]1CCC=C2CC[C@H](C[C@@]12C)C(C)=C
InChI
1S/C15H24/c1-11(2)13-8-9-14-7-5-6-12(3)15(14,4)10-13/h7,12-13H,1,5-6,8-10H2,2-4H3/t12-,13-,15+/m1/s1
InChI key
QEBNYNLSCGVZOH-NFAWXSAZSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
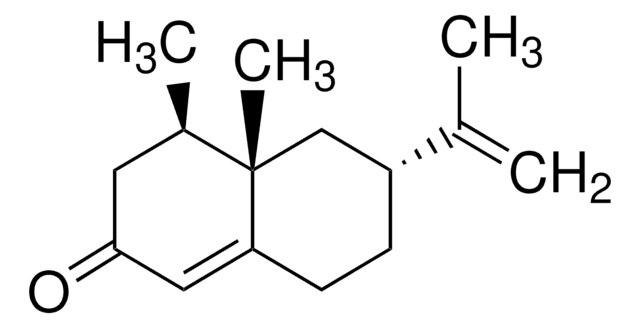
- (+)-Nootkatone (a sesquiterpene) by dark singlet oxygenation.
- Benzoyloxyvalencene by reacting with tert-butyl peroxy benzoate via Kharasch−Sosnovsky allylic oxidation method.
- (+)-Lineariifolianone, a natural product.
signalword
Danger
hcodes
Hazard Classifications
Asp. Tox. 1
wgk_germany
WGK 3
flash_point_f
212.0 °F
flash_point_c
100 °C
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-β-Farnesene; α-Huµlene; Germacrene D; (+)-Valencene; Bicyclogermacrene; (+)-δ-Cadinene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service