765953
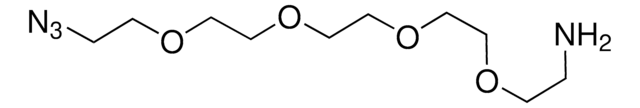
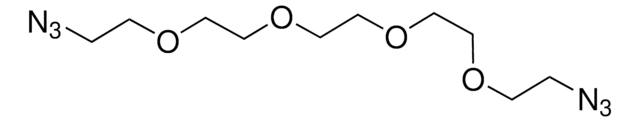
1,11-Diazido-3,6,9-trioxaundecane
Synonym(s):
Azido-PEG3-azido, Bis-PEG3-Azide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H16N6O3
CAS Number:
Molecular Weight:
244.25
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
reaction suitability
reaction type: click chemistry
reagent type: cross-linking reagent
refractive index
n20/D 1.470
bp
163-196 °C/760 mmHg
density
1.170 g/mL at 25 °C
storage temp.
−20°C
SMILES string
[N-]=[N+]=NCCOCCOCCOCCN=[N+]=[N-]
InChI
1S/C8H16N6O3/c9-13-11-1-3-15-5-7-17-8-6-16-4-2-12-14-10/h1-8H2
InChI key
SFMMXKLNFMIUCH-UHFFFAOYSA-N
Related Categories
Application
Homobifunctional PEG azide click chemistry linker. Azide functional groups will react via a copper catalyzed or strain promoted 1,3-dipolar cycloaddition click reaction with terminal alkynes and cyclooctyne derivatives to yield a stable triazole linkage.
1,11-Diazido-3,6,9-trioxaundecane may be used to synthesize:
- 1,11-bis[4-(pyren-1-ylmethoxymethyl)-1H-1,2,3-triazole-1-yl]-3,6,9-trioxaundecane, a fluorogenic chemosensor that can selective detect Hg2+ and Ag+ ions in aqueous methanol solution
- 1-amino-11-azido-3,6,9-trioxaundecane, an intermediate to synthesize tetraethylene glycol based bidentate ligand functionalized with dihydrolipoic acid and biotin (DHLA-TEG-biotin)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Design of biotin-functionalized luminescent quantum dots.
Susumu K, et al.
BioMed Research International (2007)
Highly Selective Fluorescent Sensors for Hg2+ and Ag+ Based on Bis?triazole?Coupled Polyoxyethylenes in MeOH Solution.
Hung HC, et al.
European Journal of Organic Chemistry, 2009(36), 6360-6366 (2009)
Tilman Läppchen et al.
European journal of medicinal chemistry, 89, 279-295 (2014-12-03)
Calixarene 0118 is a potent anti-angiogenic agent that effectively inhibited tumor growth in preclinical studies, and is currently being evaluated in a phase I clinical trial. We have designed two close mimetics of calixarene 0118 containing a terminal alkynyl-functional group
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)