About This Item
Recommended Products
form
liquid
Quality Level
refractive index
n/D 1.512
density
1.158 at 25 °C
storage temp.
−20°C
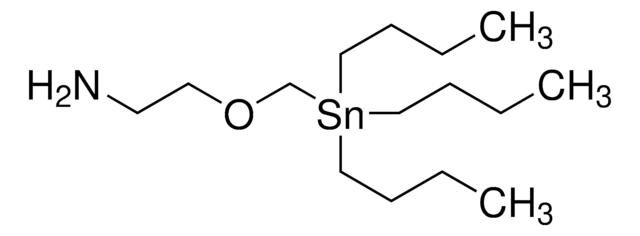
SMILES string
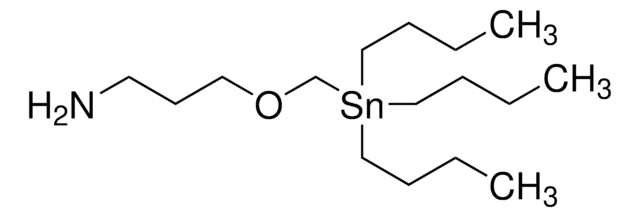
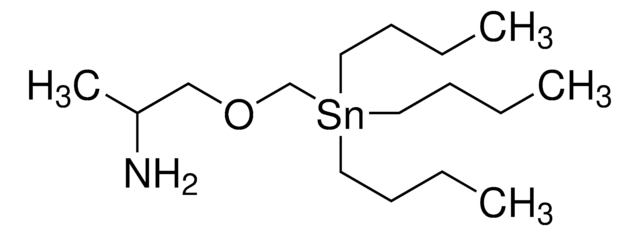
CCCC[Sn](CCCC)(CSCCN)CCCC
InChI
1S/3C4H9.C3H8NS.Sn/c3*1-3-4-2;1-5-3-2-4;/h3*1,3-4H2,2H3;1-4H2;
InChI key
DYBMHPJDHUKXRF-UHFFFAOYSA-N
Related Categories
General description
Application
Automate your N-heterocycle formation with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
Professor product portal: Jeffrey Bode Research Group
SnAP Reagents for the Synthesis of Piperazines and Morpholines
SnAP reagents for the one-step synthesis of medium-ring saturated N-heterocycles from aldehydes
SnAP Reagents for a Cross-Coupling Approach to the One-Step Synthesis of Saturated N-Heterocycles
Bespoke SnAP Reagents for the Synthesis of C-Substituted Spirocyclic and Bicyclic Saturated N-Heterocycles
related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
Saturated N-heterocyclic building blocks or SnAP Reagents are of growing importance for the convenient synthesis of medium-ring saturated N-heterocycles, including bicyclic and spirocyclic structures. SnAP reagents are stable and readily available and can be coupled with widely available aromatic, heteroaromatic, aliphatic, and glyoxylic aldehydes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service