903965
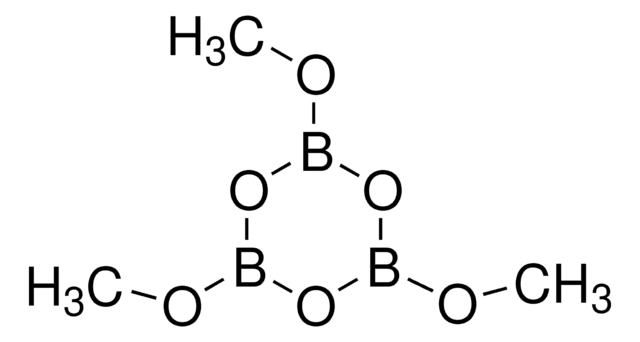
Trimethylboroxine
50% THF solution
Synonym(s):
2,4,6-Trimethyl-1,3,5,2,4,6-trioxatriborinane, 2,4,6-Trimethylboroxine, Methaneboronic anhydride, Trimethyl-1,3,5,2,4,6-trioxatriborinane
About This Item
Recommended Products
form
liquid
refractive index
n/D 1.3880
density
0.89962 g/mL
InChI
1S/C3H9B3O3/c1-4-7-5(2)9-6(3)8-4/h1-3H3
InChI key
GBBSAMQTQCPOBF-UHFFFAOYSA-N
Application
- Methylating agent for the methylation of various aromatic halides and C(sp3)−H bonds using palladium catalyst.
- Reagent in the preparation of polymer supported CBS (Corey, Bakshi, and Shibata) catalysts.
related product
signalword
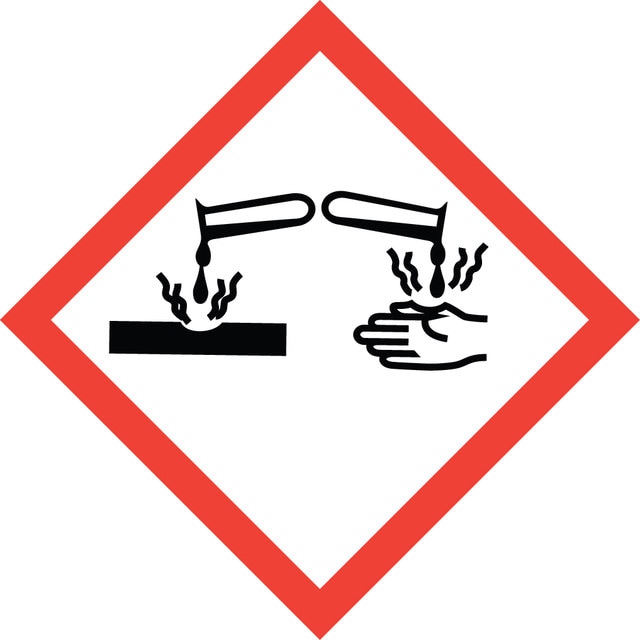
Danger
hcodes
Hazard Classifications
Carc. 2 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
supp_hazards
wgk_germany
WGK 3
flash_point_f
-5.8 °F
flash_point_c
-21 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service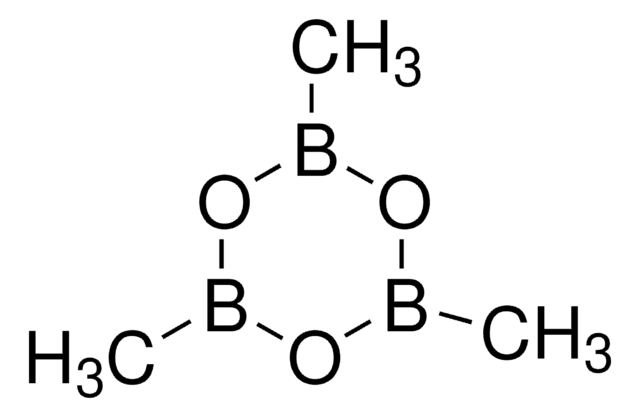
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)