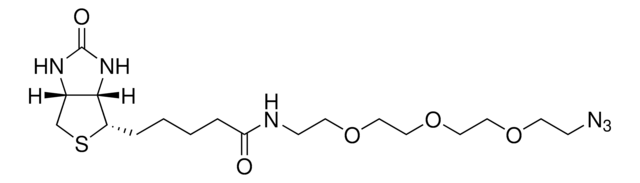
906328
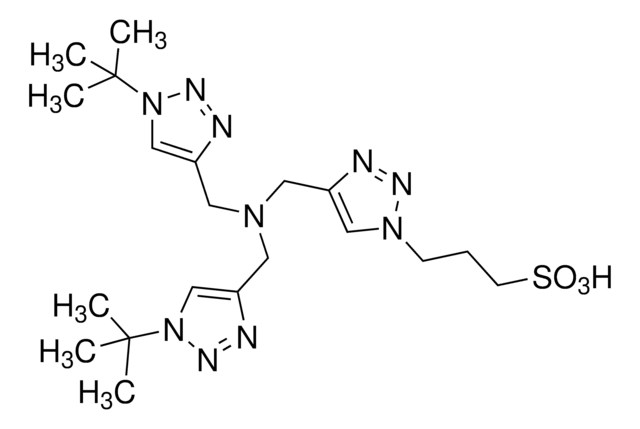
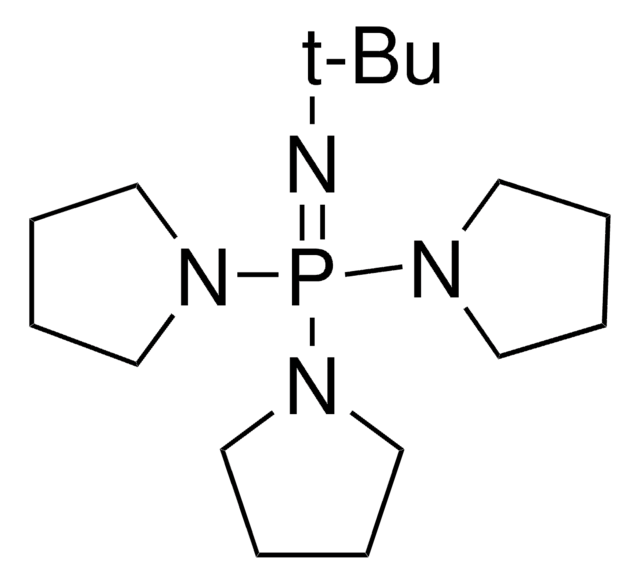
BTTAA
≥95%
Synonym(s):
2-(4-((Bis((1-(tert-butyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)acetic acid, Copper click-chemistry ligand, Water-soluble CuAAC ligand
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H30N10O2
CAS Number:
Molecular Weight:
430.51
MDL number:
UNSPSC Code:
12352200
Recommended Products
assay
≥95%
form
solid
reaction suitability
reaction type: click chemistry
availability
available only in USA
storage temp.
2-8°C
Related Categories
Application
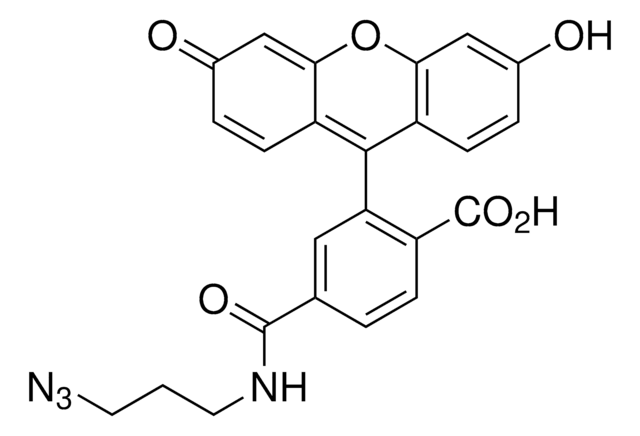
BTTAA is a next-generation, water-soluble ligand for the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) that dramatically accelerates reaction rates and suppresses cell cytotoxicity. The biocompatibility and fast kinetics of BTTAA are advancements from water-insoluble TBTA and are desirable for bio conjugation in diverse chemical biology experiments.
Other Notes
Biocompatible click chemistry enabled compartment-specific pH measurement inside E. coli
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling
Metabolic labeling of fucosylated glycoproteins in Bacteroidales species
Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling
Metabolic labeling of fucosylated glycoproteins in Bacteroidales species
Increasing the Efficacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study
signalword
Danger
hcodes
Hazard Classifications
Self-react. C
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling.
Chayasith Uttamapinant et al.
Angewandte Chemie (International ed. in English), 51(24), 5852-5856 (2012-05-05)
Samra Obeid et al.
Chemical communications (Cambridge, England), 48(67), 8320-8322 (2012-07-07)
Modified nucleotides play a paramount role in many cutting-edge biomolecular techniques. The present structural study highlights the plasticity and flexibility of the active site of a DNA polymerase while incorporating non-polar "Click-able" nucleotide analogs and emphasizes new insights into rational
Christen Besanceney-Webler et al.
Bioorganic & medicinal chemistry letters, 21(17), 4989-4992 (2011-06-17)
Members of the Bacteroidales order are among the most abundant gram-negative bacteria of the human colonic microbiota. These species decorate their cell-surface glycoproteins with fucosylated glycans, which are believed to play important roles in host intestinal colonization. Currently, there is
Junfeng Chen et al.
Journal of the American Chemical Society, 140(42), 13695-13702 (2018-09-08)
A major challenge in performing reactions in biological systems is the requirement for low substrate concentrations, often in the micromolar range. We report that copper cross-linked single-chain nanoparticles (SCNPs) are able to significantly increase the efficiency of copper(I)-catalyzed alkyne-azide cycloaddition
Yong Liang et al.
Analytical chemistry, 86(8), 3688-3692 (2014-03-25)
P450 3A4 (CYP3A4) is one of the most important isoforms in the human cytochrome P450 superfamily. It was used as an example in this proof-of-concept study in order to demonstrate an activity-based labeling and then click chemistry (CC) mediated element-tagging
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service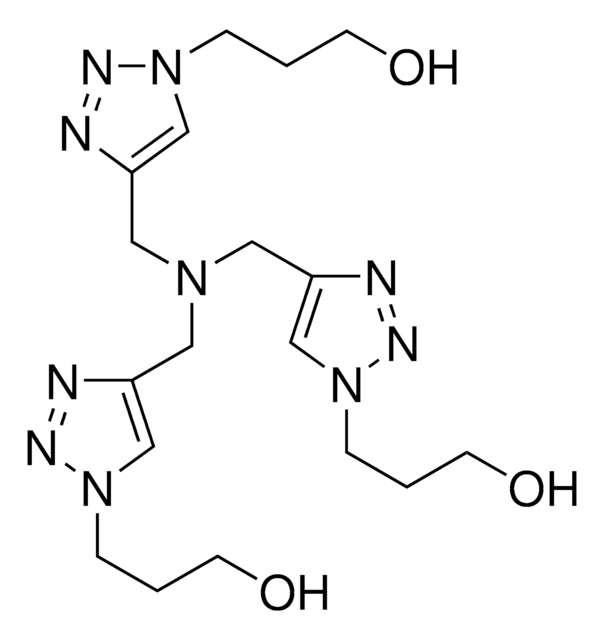
![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)