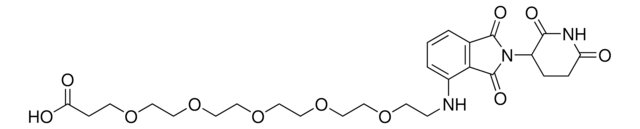
910449
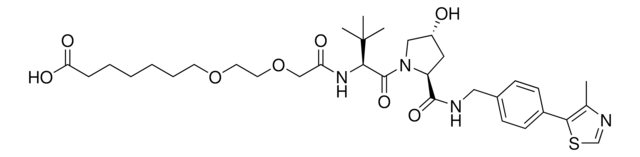
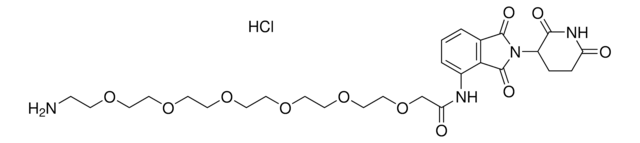
Pomalidomide-PEG2-butyl CO2H
≥95%
Synonym(s):
7-(2-(2-((2-(2,6-Dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)-2-oxoethoxy)ethoxy)heptanoic acid, Crosslinker−E3 ligase ligand conjugate, Pomalidomide conjugate, Pomalidomide-2-2-6-acid, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
Recommended Products
ligand
pomalidomide
assay
≥95%
form
powder
reaction suitability
reactivity: amine reactive
reagent type: ligand-linker conjugate
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NC(COCCOCCCCCCC(O)=O)=O)=O)NC1=O
Related Categories
Application
Other Notes
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Legal Information
related product
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service