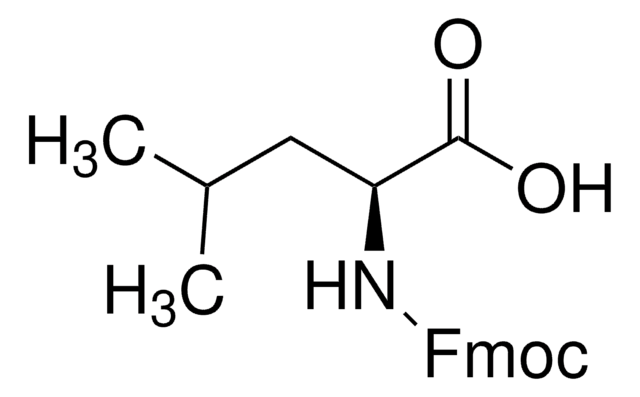
914002
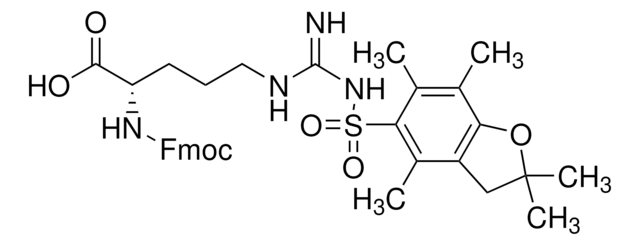
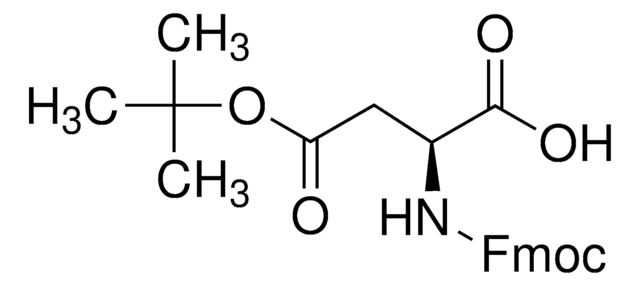
Fmoc-Asp(CSY)-OH
≥95%
Synonym(s):
(S,Z)-4-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-4-carboxy-1-cyano-1-(dimethylsulfonio)but-1-en-2-olate, Asp with cyanosulfurylide (CSY)-protected carboxylic acid, Fmoc-protected aspartic acid for minimized aspartimide formation
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C23H22N2O5S
CAS Number:
Molecular Weight:
438.50
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
Application
Fmoc-Asp(CSY)-OH is an Fmoc-protected aspartic acid residue developed in the Bode Lab that contains a cyanosulfurylide (CSY) as a carboxylic-acid protecting group that completely suppresses aspartimide formation in peptide synthesis, a long-standing challenge in peptide chemistry that occurs during Fmoc removal or peptide coupling and affects peptide yield and sequences. Amenable to SPPS, deprotection is achieved under aqueous conditions with electrophilic halogenating agents to convert the ylide to the free acid. Furthermore, the hydrophilic nature of the ylide protecting group improves overall peptide efficiency and solubility.
related product
Product No.
Description
Pricing
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kevin Neumann et al.
Nature communications, 11(1), 982-982 (2020-02-23)
Although peptide chemistry has made great progress, the frequent occurrence of aspartimide formation during peptide synthesis remains a formidable challenge. Aspartimide formation leads to low yields in addition to costly purification or even inaccessible peptide sequences. Here, we report an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service