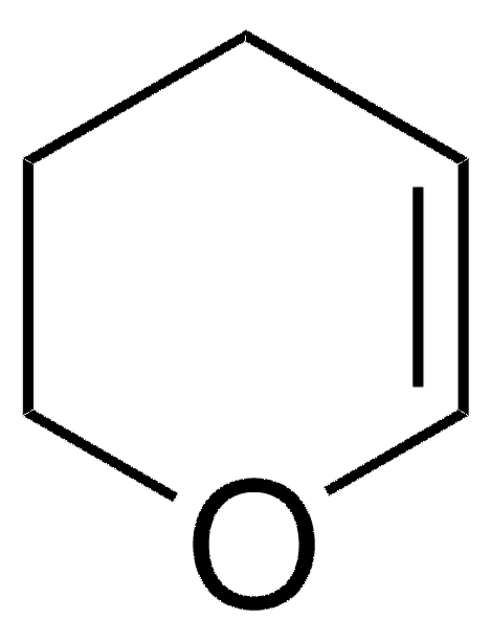
D187208
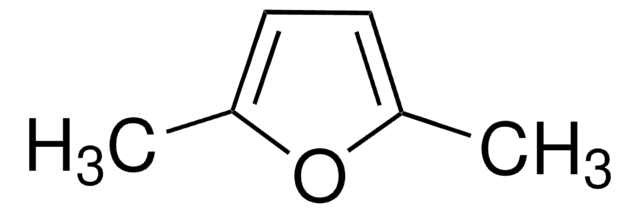
2,5-Dimethyltetrahydrofuran, mixture of cis and trans
96%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H12O
CAS Number:
Molecular Weight:
100.16
Beilstein/REAXYS Number:
102563
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
form
liquid
refractive index
n20/D 1.404 (lit.)
bp
90-92 °C (lit.)
density
0.833 g/mL at 25 °C (lit.)
SMILES string
CC1CCC(C)O1
InChI
1S/C6H12O/c1-5-3-4-6(2)7-5/h5-6H,3-4H2,1-2H3
InChI key
OXMIDRBAFOEOQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Matthew R Grochowski et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(39), 12363-12371 (2012-08-24)
Carbohydrates, such as fructose, can be fully dehydroxylated to 2,5-dimethyltetrahydrofuran (DMTHF), a valuable chemical and potential gasoline substitute, by the use of a dual catalytic system consisting of HI and RhX(3) (X=Cl, I). A mechanistic study has been carried out
M S Akhlaq et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 51(1), 91-102 (1987-01-01)
Thiyl radicals (RS) formed by the reaction of radiolytically generated OH radicals with thiols, e.g. 1,4-dithiothreitol (DTT), react with cis- and trans-2,5-dimethyltetrahydrofuran by abstracting an H atom in the alpha-position to the ether function (k approximately equal to 5 X
Weiran Yang et al.
ChemSusChem, 3(5), 597-603 (2010-05-04)
Existing technologies to produce liquid fuels from biomass are typically energy-intensive, multistep processes. Many of these processes use edible biomass as starting material. Carbohydrates, such as mono- and polysaccharides and cellulose, typically constitute 50-80% of plant biomass. Herein, we report
A S Anderson et al.
The Journal of organic chemistry, 65(15), 4648-4654 (2000-08-26)
2'-Deoxy-5-methyleneuridin-5-yl (1) is produced in a variety of DNA damage processes and is believed to result in the formation of lesions that are mutagenic and refractory to enzymatic repair. 2'-Deoxy-5-methyleneuridin-5-yl (1) was independently generated under anaerobic conditions via Norrish Type
John M Simmie
The journal of physical chemistry. A, 116(18), 4528-4538 (2012-04-13)
The enthalpies of formation, entropies, specific heats at constant pressure, enthalpy functions, and all carbon-hydrogen and carbon-methyl bond dissociation energies have been computed using high-level methods for the cyclic ethers (oxolanes) tetrahydrofuran, 2-methyltetrahydrofuran, and 2,5-dimethyltetrahydrofuran. Barrier heights for hydrogen-abstraction reactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service