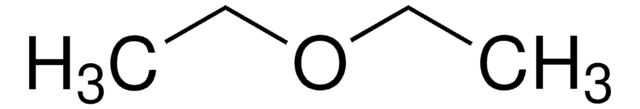
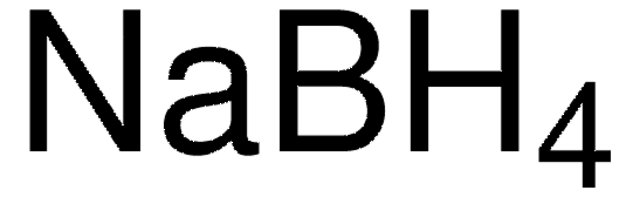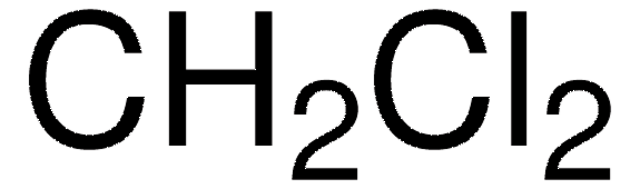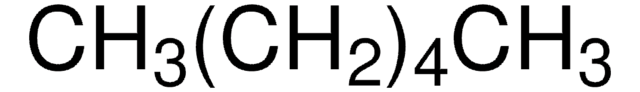F1506
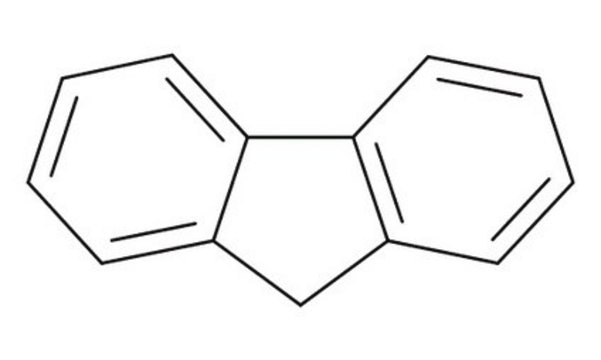
9-Fluorenone
98%
Synonym(s):
9H-Fluorene-9-one, Fluoren-9-one (8CI)
About This Item
Recommended Products
Quality Level
assay
98%
bp
342 °C (lit.)
mp
80-83 °C (lit.)
SMILES string
O=C1c2ccccc2-c3ccccc13
InChI
1S/C13H8O/c14-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1-8H
InChI key
YLQWCDOCJODRMT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Synthesis of host for the blue and green phosphorescent organic light emitting diodes (PHOLEDs).
- Synthesis of fluorene-based molecular motors.
- Synthesis of open-shell Chichibabin′s hydrocarbons as potential organic spintronic materials.
- It also acts as a sensitizer in the formation of picene via photosensitization of 1,2-di(1-naphthyl)ethane.
signalword
Warning
hcodes
Hazard Classifications
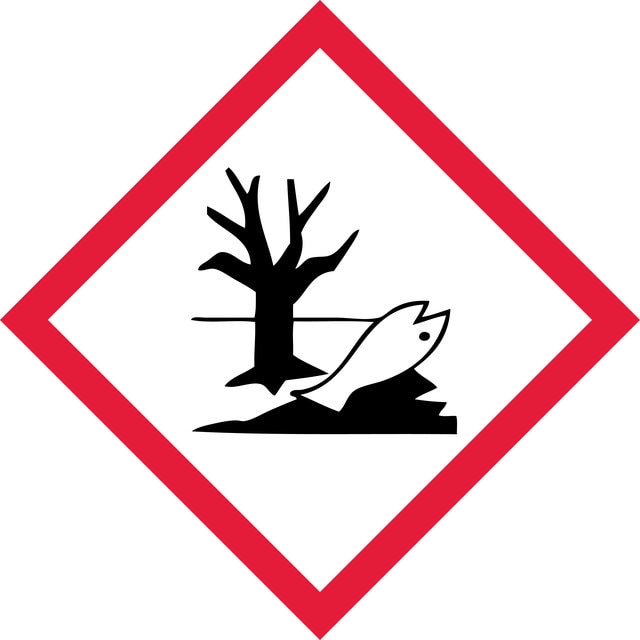
Aquatic Chronic 2 - Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
325.4 °F
flash_point_c
163 °C
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Analysis of heavy metals and metals in cannabis and hemp containing personal care products and cosmetics include sample preparation by grinding, preparation of calibration solutions for a standard addition approach, a microwave digestion protocol and subsequent ICP-MS analysis.
Learn about the four membrane-bound protein complexes that make up the electron transport chain metabolic pathway supplying energy as ATP for cellular respiration.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service