N257
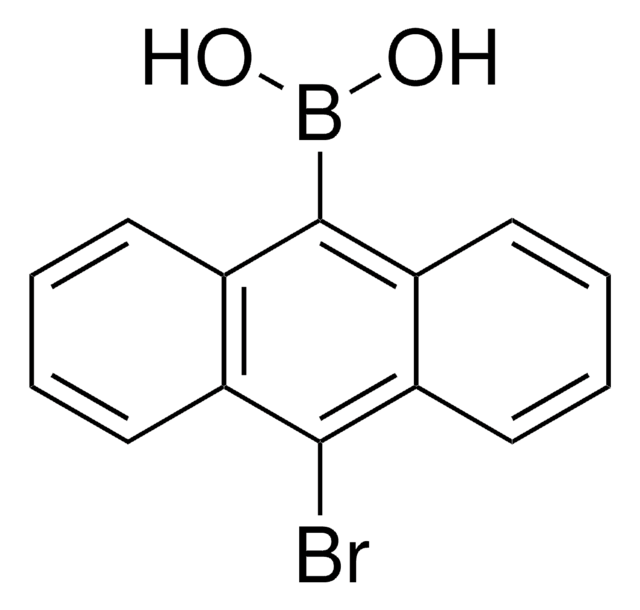
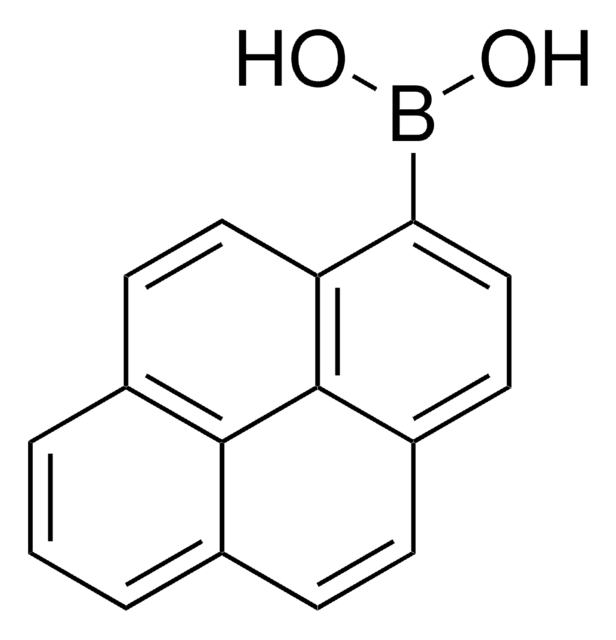
Naphthalene-1-boronic acid
≥95.0%
Synonym(s):
α-Naphthaleneboronic acid, α-Naphthylboronic acid, 1-Naphthaleneboronic acid, 1-Naphthylboronic acid, 1-naphthalenyl-boronic acid, NSC 78936
About This Item
Recommended Products
Quality Level
assay
≥95.0%
form
crystals
mp
208-214 °C (lit.)
SMILES string
OB(O)c1cccc2ccccc12
InChI
1S/C10H9BO2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,12-13H
InChI key
HUMMCEUVDBVXTQ-UHFFFAOYSA-N
Related Categories
Application
- Methyl 5-bromo-2-(naphthalene-1-yl)benzoate, which is a starting material for the preparation of 9-anthracene-spirobenzofluorene host materials.
- A bifunctional polymer used for the hydrolysis of cellulose.
- 9-(Naphthalene-1-yl)anthracene, which is a key intermediate for the preparation of anthracene derived molecular glass having blue emission.
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service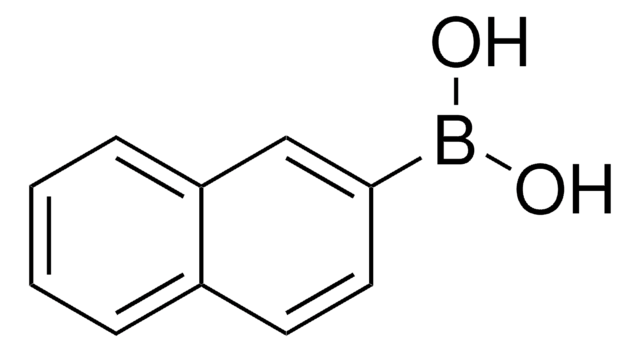
![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)