P73404
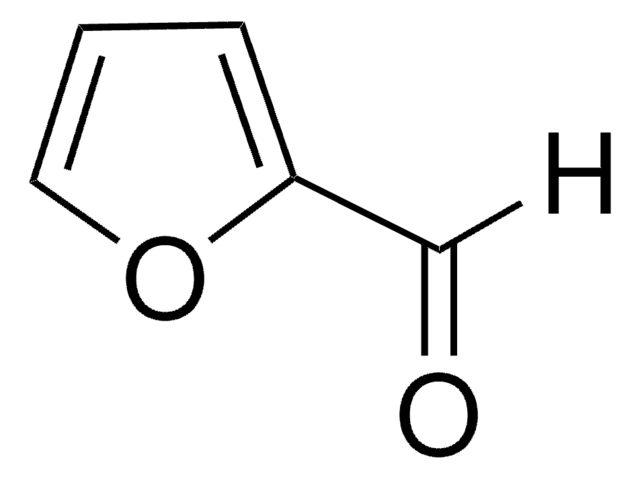
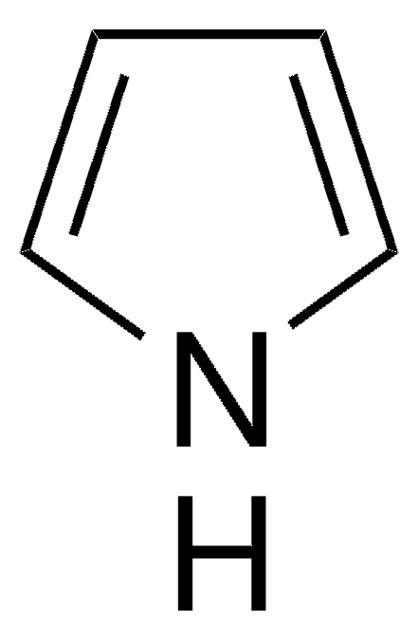
Pyrrole-2-carboxaldehyde
98%
Synonym(s):
2-Formylpyrrole
About This Item
Recommended Products
Quality Level
assay
98%
form
crystals
bp
217-219 °C (lit.)
mp
43-46 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccc[nH]1
InChI
1S/C5H5NO/c7-4-5-2-1-3-6-5/h1-4,6H
InChI key
ZSKGQVFRTSEPJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa.: This study used pyrrole-2-carboxaldehyde to develop a fluorescent nanoparticle probe based on pyrimidine for detecting Pseudomonas aeruginosa, enhancing diagnostic capabilities in microbiology (Kaur G et al., 2015).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
224.6 °F - closed cup
flash_point_c
107 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service