W304506
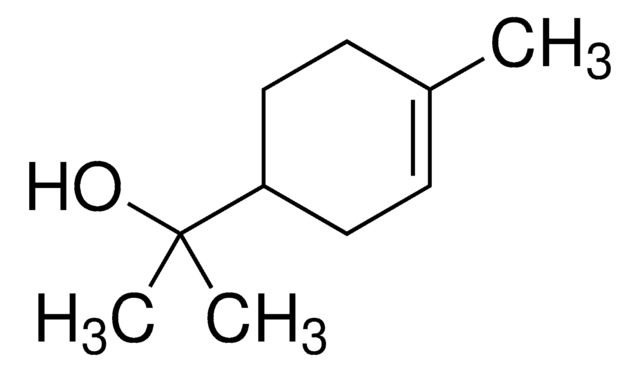
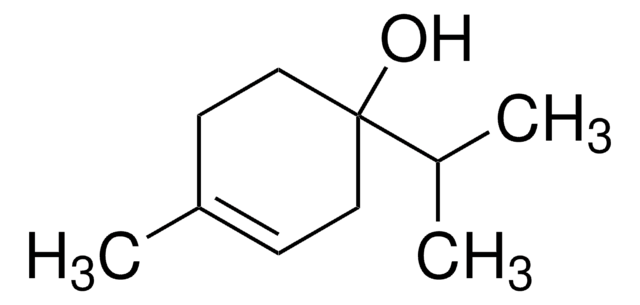
Terpineol
mixture of isomers, 96%, FG
Synonym(s):
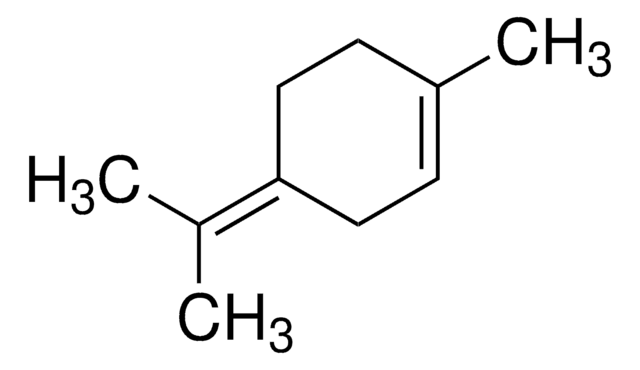
α,α,4-trimethyl-3-cyclohexene-1-methanol, 2-(4-methyl-3-cyclohexen-1-yl)-2-propanol, p-menth-1-en-8-ol
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
FDA 21 CFR 178.1010
assay
96%
form
viscous liquid
composition
d-terpineol, <1.0%
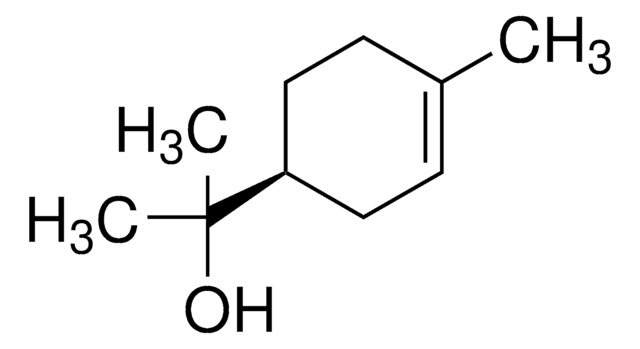
a-terpineol, ≥55.0%
b-terpineol, <10.0% (cis)
b-terpineol, <13.0% (trans)
g-terpineol, <23.0%
bp
213-218 °C (lit.)
solubility
ethanol: soluble 1.25ml/10ml, clear to slightly hazy, colorless to light yellow (50% ethanol)
ethanol: soluble 2.5ml/10ml, clear to slightly hazy, colorless to light yellow
ethanol: soluble 5mL/10mL, clear to slightly hazy, colorless to light yellow (70% Ethanol)
density
0.934 g/mL at 20 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
terpineol (mixture of isomers), α-terpineol
organoleptic
lilac; woody; floral; citrus; pine
SMILES string
CC(=C)C1CCC(C)(O)CC1.CC2=CCC(CC2)C(C)(C)O
InChI
1S/2C10H18O/c1-8-4-6-9(7-5-8)10(2,3)11;1-8(2)9-4-6-10(3,11)7-5-9/h4,9,11H,5-7H2,1-3H3;9,11H,1,4-7H2,2-3H3
InChI key
MGABGZGUMNDCSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
No data available
flash_point_c
No data available
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocols
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
-β-Farnesene; α-Huµlene; Germacrene D; (+)-Valencene; Bicyclogermacrene; (+)-δ-Cadinene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service