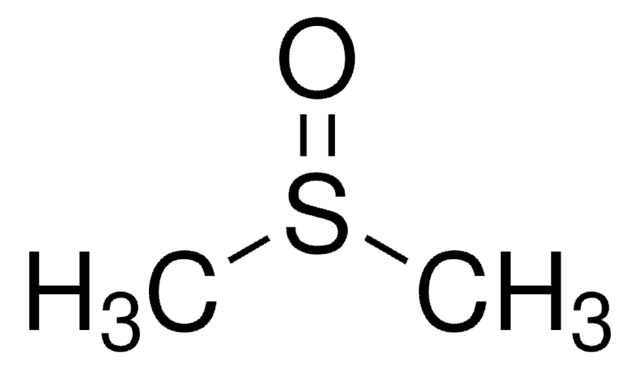
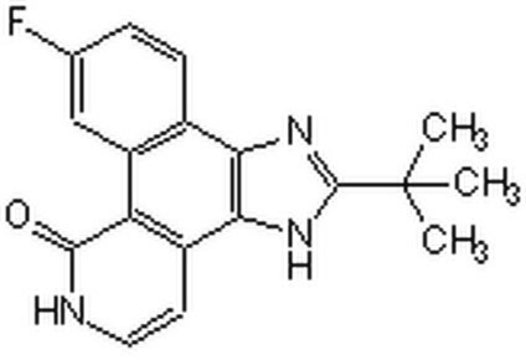
L-030
Lurasidone hydrochloride solution
1.0 mg/mL in methanol (as free base), ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol (as free base)
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
Cl.O=C1[C@@H]2[C@H]3CC[C@H](C3)[C@@H]2C(=O)N1C[C@@H]4CCCC[C@H]4CN5CCN(CC5)c6nsc7ccccc67
InChI
1S/C28H36N4O2S.ClH/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26;/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2;1H/t18-,19+,20-,21-,24+,25-;/m0./s1
InChI key
NEKCRUIRPWNMLK-SCIYSFAVSA-N
Gene Information
human ... DRD2(1813) , HTR2A(3356)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- LurasiDonein Bipolar Depression Research: A study explored the pharmacodynamic properties of lurasidone, hypothesizing its efficacy in acute bipolar depression. This research provides a deep dive into the mechanistic actions of lurasidone, enhancing understanding in neuropharmacological studies and aiding in the development of more effective treatments for bipolar disorder (Fountoulakis et al., 2024).
- Quantification of LurasiDonein Clinical Samples: Development and validation of a liquid chromatography-tandem mass spectrometry method for quantifying lurasiDonein dried blood spot samples was reported. This method facilitates easier and less invasive monitoring of lurasiDonelevels in patients, crucial for effective pharmacological research and ensuring therapeutic efficacy in treatment regimes (Rajadhyaksha and Londhe, 2023).
- Novel Methodologies in Clinical Trials: Research introduced a novel method for deriving adverse event prevalence in randomized controlled trials, which could potentially improve the understanding of the benefit-risk ratio of drugs including lurasidone. This approach is particularly relevant for drug labels and regulatory submissions, ensuring safer and more effective clinical outcomes (Piacentino et al., 2024).
- Pharmacological Properties of Lurasidone: A study investigated how lurasiDoneblocks the voltage-gated potassium channels of coronary arterial smooth muscle cells, offering insights into its broader pharmacological impacts. This research is vital for assessing potential cardiovascular side effects and optimizing dosing strategies to mitigate risks in patients treated with lurasiDone(Zhuang et al., 2023).
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes
wgk_germany
WGK 1
flash_point_f
closed cup
flash_point_c
closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service