M-065
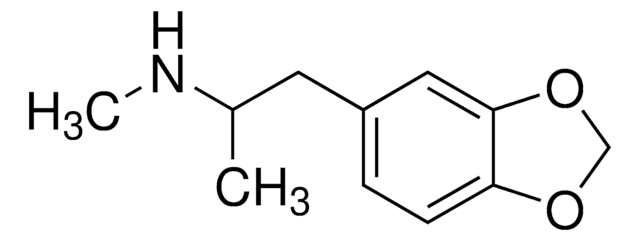
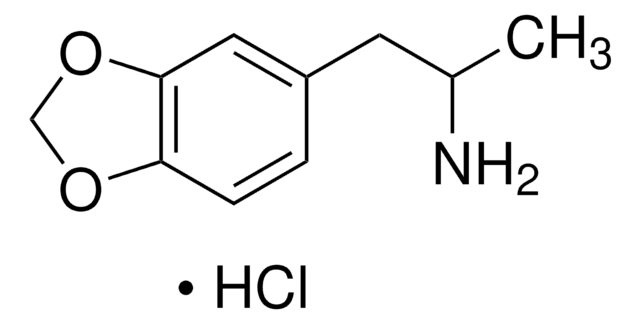
(±)-MDEA solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
Synonym(s):
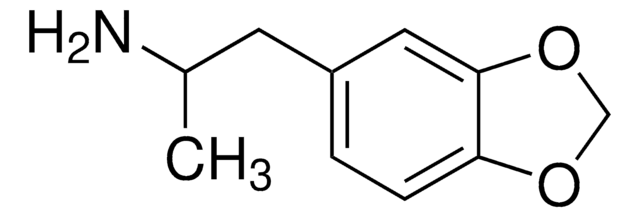
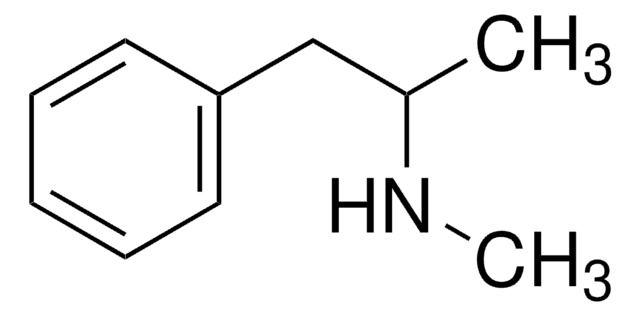
(±)-3,4-Methylenedioxyethylamphetamine
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule D (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
2-8°C
SMILES string
CC(CC1=CC=C(OCO2)C2=C1)NCC
InChI
1S/C12H17NO2/c1-3-13-9(2)6-10-4-5-11-12(7-10)15-8-14-11/h4-5,7,9,13H,3,6,8H2,1-2H3
InChI key
PVXVWWANJIWJOO-UHFFFAOYSA-N
General description
Application
- (±)-MDEA Solution for Neurotransmitter Research: Utilized extensively in the study of serotonin release mechanisms, (±)-MDEA-D3 provides a robust tool for examining the biochemical pathways involved in neurotransmitter dynamics, essential for advancements in neuropharmacology (Wen et al., 2024).
- Racemic MDEA Reagent for Pharmacological Studies: This solution is crucial for the synthesis and pharmacological evaluation of new psychoactive substances, enabling researchers to delineate the metabolic and pharmacodynamic profiles of novel therapeutic agents (Boucenna et al., 2023).
- High-Purity (±)-MDEA-D3 Reference Material: As a reference standard, this high-purity material is vital for calibrating analytical instruments like LC-MS/MS, ensuring accuracy and reproducibility in quantitative drug analysis and forensic toxicology (Ghorbani et al., 2020).
- (±)-MDEA-D3 Internal Standard for LC-MS/MS: This deuterated version of MDEA serves as an internal standard in chromatographic analyses, enhancing the precision of quantitative measurements in complex biological matrices (Tian et al., 2021).
- (±)-MDEA-D3 Solution for Pharmaceutical Cannabinoid Profiling: Employed in cannabinoid biosynthesis research, (±)-MDEA-D3 aids in the profiling and characterization of cannabinoids, supporting the development of cannabis-based pharmaceuticals with targeted therapeutic effects (Irani et al., 2018).
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
49.5 °F - closed cup
flash_point_c
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service