1.01115
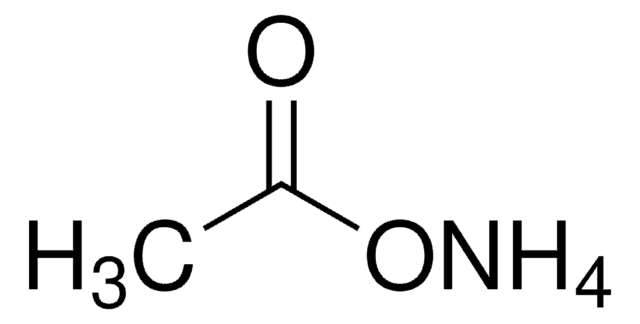
Ammonium acetate
EMPLURA®
Synonym(s):
Ammonium acetate
About This Item
Recommended Products
Quality Level
product line
EMPLURA®
assay
≥96.0% (alkalimetric)
form
solid
ign. residue
≤0.02% (as sulfate)
pH
6.7-7.3 (25 °C, 50 g/L in H2O)
mp
114 °C
solubility
1480 g/L
density
1.17 g/cm3 at 20 °C
bulk density
410 kg/m3
anion traces
chloride (Cl-): ≤0.002%
sulfate (SO42-): ≤0.01%
cation traces
Fe: ≤0.001%
heavy metals (as Pb): ≤0.0005%
storage temp.
15-25°C
InChI
1S/C2H4O2.H3N/c1-2(3)4;/h1H3,(H,3,4);1H3
InChI key
USFZMSVCRYTOJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- One-Pot, Four-Component Reaction for the Design, Synthesis, and SAR Studies of Novel Pyridines for Insecticidal Bioefficacy Screening against Cowpea Aphid (Aphis craccivora): This study explores the application of ammonium acetate in facilitating the synthesis of novel pyridine derivatives, evaluated for their insecticidal properties against Aphis craccivora (Alkorbi et al., 2024).
- A green analytical method for the simultaneous determination of 17 perfluoroalkyl substances (PFAS) in human serum and semen by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS): This research demonstrates the use of ammonium acetate in UPLC-MS/MS for detecting PFAS in biological samples, highlighting its role in environmental and health-related analytical chemistry (Di Giorgi et al., 2024).
- Innovation of 6-sulfonamide-2H-chromene derivatives as antidiabetic agents targeting α-amylase, α-glycosidase, and PPAR-γ inhibitors with in silico molecular docking simulation: Ammonium acetate was utilized in the synthesis process of new chromene derivatives, showing potential as antidiabetic agents by inhibiting α-amylase and α-glycosidase activities (Thabet et al., 2024).
- Regulation of Tetramethylpyrazine Formation by the Phenolics-Fenton Coupled Redox Cycling System: This article presents a study on the role of ammonium acetate in the formation of tetramethylpyrazine via redox cycling, an important reaction in food chemistry and possibly in synthesizing complex organic molecules (Xu et al., 2024).
- Simultaneous determination of unecritinib (TQ-B3101) and its active metabolite crizotinib in rat plasma by LC-MS/MS: An application to pharmacokinetic studies: This paper details the use of ammonium acetate in enhancing LC-MS/MS methodologies for pharmacokinetic studies, exemplifying its critical role in the analysis of pharmaceutical compounds (Wang et al., 2024).
Analysis Note
Identity: passes test
pH-value (5 %; water): 6.0 - 7.5
Chloride (Cl): ≤ 0.002 %
Sulfate (SO₄): ≤ 0.01 %
Heavy metals (as Pb): ≤ 0.0005 %
Fe (Iron): ≤ 0.001 %
Residue on ignition (as sulfate): ≤ 0.02 %
Water: ≤ 2.5 %
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service