1.05463
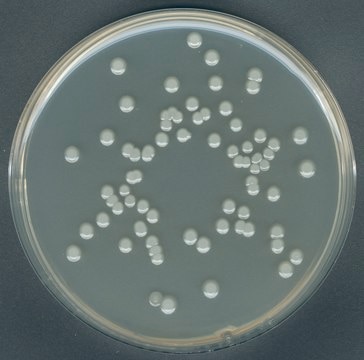
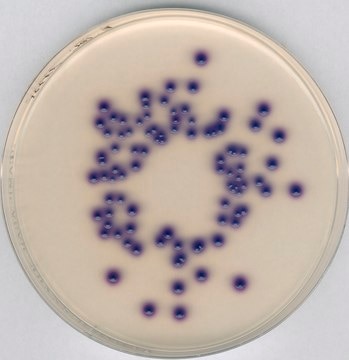
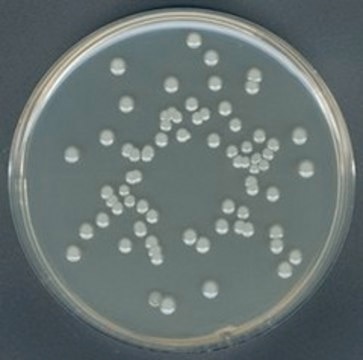
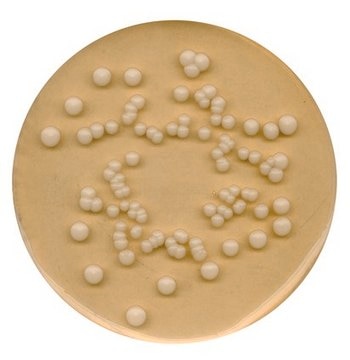
GranuCult® Plate Count agar
ISO 4833, ISO 17410, FDA (BAM), for aerobic bacteria
Synonym(s):
Aerobic Plate Count Agar, Casein-peptone dextrose yeast agar, Standard Methods Agar
About This Item
Recommended Products
agency
APHA
ISO 17410
ISO 4833
Quality Level
reg. compliance
FDA (BAM)
form
medium granules (dehydrated (DCM))
manufacturer/tradename
GranuCult®
technique(s)
microbiological culture: suitable
pH
7.0 (30 °C, 22 g/L in H2O, after autoclaving)
solubility
22.5 g/L
bulk density
560 kg/m3
application(s)
food and beverages
microbiology
storage temp.
15-25°C
suitability
aerobic bacteria (total)
Related Categories
General description
Application
Linkage
Footnote
The designations basic, plus, or prime are added to indicate the quality control level, from basic quality control to standard QC plus to prime for full regulatory compliance.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The different types of culture media, that are used to grow microorganisms in the laboratory for quality control, are classified by several criteria, such as consistency, composition, or selectivity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service