1.08212
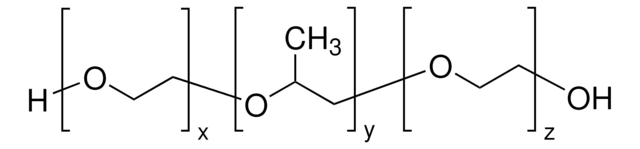
Parteck® PLX 188 (Poloxamer 188)
(stabilized with 70ppm BHT), EMPROVE® ESSENTIAL, Ph. Eur., NF
Pharma Manufacturing
Synonym(s):
Poloxamer 188
About This Item
Recommended Products
biological source
synthetic
Quality Level
agency
NF
Ph. Eur.
product line
EMPROVE® ESSENTIAL
bp
>150 °C
mp
55 °C
bulk density
1050 kg/m3
storage temp.
2-25°C
InChI
1S/C3H6O.C2H4O/c1-3-2-4-3;1-2-3-1/h3H,2H2,1H3;1-2H2
InChI key
RVGRUAULSDPKGF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
As part of our Emprove® Program, our raw materials are offered with extensive documentation facilitating compliance of your pharma and biopharma product, full supply chain transparency and risk mitigation. Our SAFC® portfolio of high-quality products for biopharmaceutical and pharmaceutical formulation and production withstands strict quality control procedures and is produced according to applicable cGMP guidelines.
Application
Features and Benefits
- Hydrophilic, water-soluble tablet lubrication
- Improved dissolution rates for poorly soluble DCS class IIa molecules
- Specified particle size distribution optimized for solid application performance
- Regulatory support through compliance with Ph. Eur. and USP-NF, going beyond current requirements with specitied formaldehyde content
Legal Information
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service