1.46628
Sterile Isopropyl Myristate (IPM)
ready-to-use, bottle volume 360 mL , filling volume
Synonym(s):
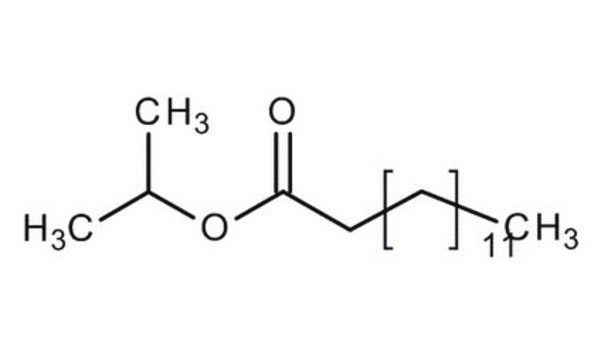
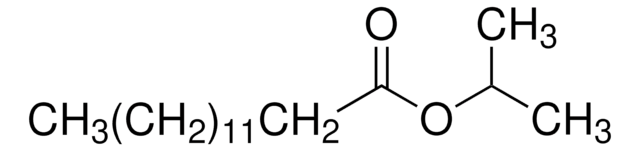
Isopropylmyristate, Isopropylmyristate, Tetradecanoic acid isopropyl ester, Myristic acid isopropyl ester, Sterile IPM, Tetradecanoic acid isopropyl ester, Myristic acid isopropyl ester
About This Item
Recommended Products
agency
EP 2.6.13
JP 4.06
USP 71
Quality Level
vapor pressure
<1 hPa ( 20 °C)
description
Suitable for dilution of viscous products to facilitate membrane filtration
sterility
sterile; γ-irradiated
form
liquid
autoignition temp.
>300 °C
shelf life
12 mo.
feature
closure type crimp cap with septum
ready-to-use
packaging
bottle of
pkg of (360 ml in 500 ml bottle with red flip cap and septum (6 bottles per box))
bottle capacity
500 mL
bottle volume
360 mL , filling volume
bp
140 °C/3 hPa
transition temp
flash point >150 °C
solubility
<0.05 mg/L
density
0.85 g/cm3 at 20 °C
application(s)
cosmetics
food and beverages
pharmaceutical
sterility testing
storage temp.
15-25°C
suitability
nonselective for
InChI
1S/C17H34O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-17(18)19-16(2)3/h16H,4-15H2,1-3H3
InChI key
AXISYYRBXTVTFY-UHFFFAOYSA-N
Related Categories
General description
Application
Features and Benefits
- Preparing IPM for microbiological testing is a hurdle for any QC laboratory. Sterility is usually achieved with a long and difficult filtration process. Our Sterile IPM is sterilized by Gamma-irradiation and can be used as any ready-to-use rinse fluid or culture media.
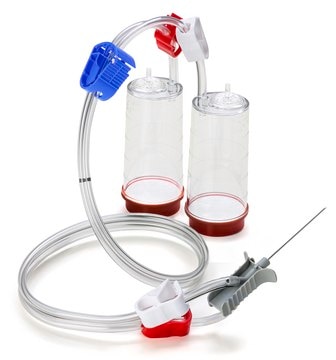
- For sterility testing sterile IPM should be used in combination with Our Steritest® NEO “Green base” device (TZHVSL210).
Legal Information
configured for
Storage Class
10 - Combustible liquids
wgk_germany
awg
flash_point_f
302.0 °F
flash_point_c
> 150 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Regulatory compliant membrane filtration sterility testing devices to ensure the safety of your pharmaceutical products.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service