220551
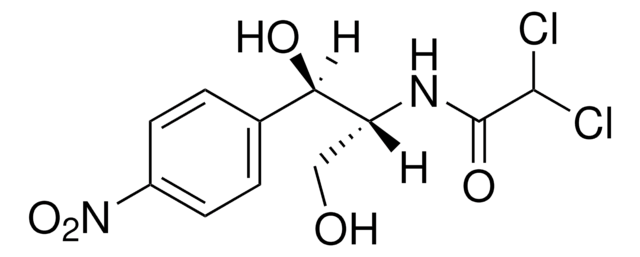
Chloramphenicol
Chloramphenicol, CAS 56-75-7, is a synthetic bacteriostatic antibiotic that inhibits the translation of RNA by blocking the peptidyltransferase reaction on ribosomes.
Synonym(s):
Chloramphenicol
About This Item
Recommended Products
Quality Level
assay
≥97% (by Assay)
form
crystalline powder
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
color
gray to off-white
solubility
water: 2.5 mg/mL
methanol: 35 mg/mL
ethanol: soluble
shipped in
ambient
storage temp.
10-30°C
InChI
1S/C11H12Cl2N2O5/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20/h1-4,8-10,16-17H,5H2,(H,14,18)
InChI key
WIIZWVCIJKGZOK-UHFFFAOYSA-N
Related Categories
General description
Biochem/physiol Actions
trasnslation of RNA
Other Notes
Lu, J., and Jiang, C. 1993. Biochem. Biophys. Res. Commun.196, 12.
Saltarelli, M.J., et al. 1993. Virol.197, 35.
Holt, J.T. 1992. Ann. N.Y. Acad. Sci.660, 88.
Maniatis, T., et al. 1989. In Molecular Cloning, A Laboratory Manual, Second Edition. Cold Spring Harbor, NY, p. 1.6, A.6.
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Repr. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Exploring genotoxicity and DNA damage through multiplexing with MILLIPLEX® multiplex genotoxicity assays using Luminex® xMAP® technology enables the high-throughput measurement of phosphorylation levels of multiple proteins simultaneously and reduces sample volume, time, and cost.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service