475941
Myokinase, Yeast
Synonym(s):
Myokinase, Yeast, Adenylate Kinase, ATP:AMP Phosphotransferase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352202
Recommended Products
form
lyophilized
Quality Level
specific activity
≥10 units/mg solid
≥200 U/mg
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
pI
5.7
solubility
water: 1 mg/mL
foreign activity
ATPase ≤0.01%
Phosphoglycerate kinase ≤0.1%
shipped in
wet ice
storage temp.
−20°C
General description
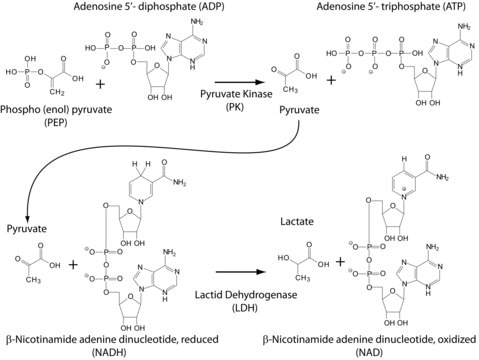
Native, yeast myokinase. Myokinase reversibly transfers phosphate from ATP to AMP, thus forming two molecules of ADP. The enzyme is specific for adenine nucleotides and catalyzes the reaction only in the presence of a divalent metal ion (e.g. Mg2+, Ca2+, Co2+, Mn2+, or Ni2+).
Native, yeast myokinase. Myokinase reversibly transfers phosphate from ATP to AMP, thus forming two molecules of ADP. The enzyme is specific for adenine nucleotides and catalyzes the reaction only in the presence of a divalent metal ion (e.g. Mg2+, Ca2+, Co2+, Mn2+, or Ni2+).
Note: 1 KU = 1000 units.
Warning
Toxicity: Standard Handling (A)
Unit Definition
One unit is defined as the amount of enzyme that will convert 1.0 µmol ATP and 1.0 µmol AMP to 2 µmol ADP per min at 25°C, pH 7.5. Note: 1 KU = 1000 units.
Physical form
Lyophilized from potassium phosphate buffer.
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Other Notes
Makarchikov, A.F., et al. 2002. Biochem. Biophys. Acta.1592, 117.
Schricker, R., et al. 2002. J. Biol. Chem.277, 28757.
Magdolen, V., et al. 1987. Curr. Genet.12, 405.
Tomasselli, A.G., et al. 1986. Eur. J. Biochem.155, 111.
Ito, Y., et al. 1980. Eur. J. Biochem.105, 85.
Schricker, R., et al. 2002. J. Biol. Chem.277, 28757.
Magdolen, V., et al. 1987. Curr. Genet.12, 405.
Tomasselli, A.G., et al. 1986. Eur. J. Biochem.155, 111.
Ito, Y., et al. 1980. Eur. J. Biochem.105, 85.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V Magdolen et al.
Current genetics, 12(6), 405-411 (1987-01-01)
The structural gene for yeast adenylate kinase (AKY) has been isolated and analyzed with respect to its nucleotide sequence. Southern and northern analyses imply that the gene is single copy and is transcribed into an mRNA of about 1,100 bases.
Y Ito et al.
European journal of biochemistry, 105(1), 85-92 (1980-03-01)
An improved homogeneous preparation of adenylate kinase (ATP:AMP phosphotransferase, ATP + AMP in equilibrium 2 ADP) from baker's yeast was attained by extraction using ethyl acetate and successive column chromatography on Affi-Gel blue, Sephadex G-100, phosphocellulose and Sephacryl S-200. The
A G Tomasselli et al.
European journal of biochemistry, 155(1), 111-119 (1986-02-17)
The complete amino acid sequence of cytosolic adenylate kinase (MgATP + AMP----MgADP + ADP) from baker's yeast has been determined. Tryptic and clostripaic cleavage of the protein yielded 27 and 10 fragments, respectively. They were sequenced with either a solid-phase
Roland Schricker et al.
The Journal of biological chemistry, 277(32), 28757-28764 (2002-06-05)
Yeast adenylate kinase (Aky2p, Adk1p) occurs simultaneously in cytoplasm and mitochondrial intermembrane space. It has no cleavable mitochondrial targeting sequence, and the signal for mitochondrial import and submitochondrial sorting is largely unknown. The extreme N terminus of Aky2p is able
Alexander F Makarchikov et al.
Biochimica et biophysica acta, 1592(2), 117-121 (2002-10-16)
Thiamine triphosphate (ThTP) is found at low concentrations in most animal tissues and it may act as a phosphate donor for the phosphorylation of proteins, suggesting a potential role in cell signaling. Two mechanisms have been proposed for the enzymatic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service