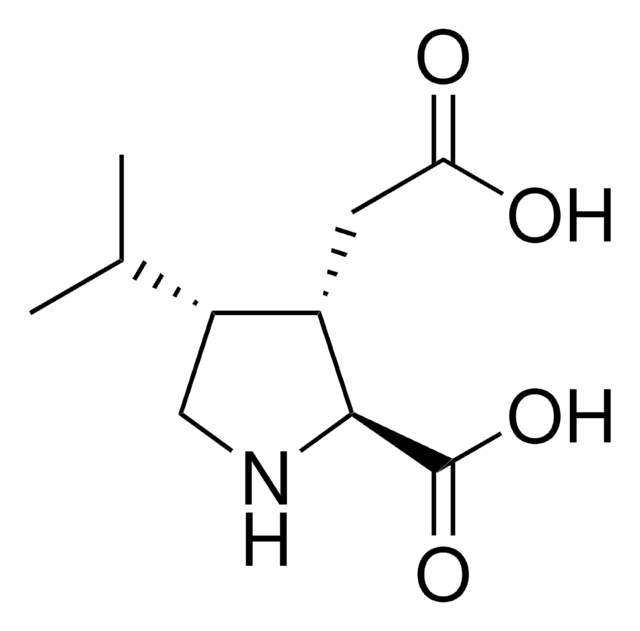
5.08942
Prokineticin 2 Antagonist, PKRA7
Synonym(s):
Prokineticin 2 Antagonist, PKRA7, PROK2 Antagonist, PKRA7, Bv8 Antagonist, PKRA7, PK2 Antagonist, PKRA7
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C27H34ClFN2O4
CAS Number:
Molecular Weight:
505.02
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
assay
≥98% (HPLC)
Quality Level
form
semisolid
potency
5 nM IC50
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
colorless
solubility
DMSO: 100 mg/mL
storage temp.
2-8°C
General description
A cell permeable, brain permeant 3-pyrrolidine carboxamide derivative that acts as an antagonist of polypeptide chemokine prokineticin 2 (PK2) receptor (Bv8, PROK2; IC50 = 5 nM and 8.2 nM for PKR1 and PKR2, respectively). Suppresses the growth of D456MG glioma cell xenografts in nude mice by reducing blood vessel density and blocking angiogenesis via inhibition of PKR1 and PKR2 expressed on endothelial cells. Shown to suppress the growth of AsPC1 and CFPac-1 pancreatic tumor cells in xenograft model of nude mice by reducing macrophage migration and inflitration. Reduces the expression of CCL27, CCR10, CCR4, CCR5, and CCR6 pro-migratory chemokines and chemokine receptors in macrophages. Enhances the efficacy of standard temomolomide in the treatment of glioblastoma and gemcitabine in the treatment of pancreatic cancer in xenograft nude mice models.
A cell permeable, brain permeant 3-pyrrolidine carboxamide derivative that acts as an antagonist of polypeptide chemokine prokineticin 2 (PK2) receptor (Bv8, PROK2; IC50 = 5 nM and 8.2 nM for PKR1 and PKR2, respectively). Suppresses the growth of D456MG glioma cell xenografts in nude mice by reducing blood vessel density and blocking angiogenesis via inhibition of PKR1 and PKR2 expressed on endothelial cells. Shown to suppress the growth of AsPC1 and CFPac-1 pancreatic tumor cells in xenograft model of nude mice by reducing macrophage migration and inflitration. Reduces the expression of CCL27, CCR10, CCR4, CCR5, and CCR6 pro-migratory chemokines and chemokine receptors in macrophages. Enhances the efficacy of standard temomolomide in the treatment of glioblastoma and gemcitabine in the treatment of pancreatic cancer in xenograft nude mice models.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Biochem/physiol Actions
Primary Target
PROK2
PROK2
Reversible: yes
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Other Notes
Curtis, V., et al. 2013. PLoS One.8, e54916.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service