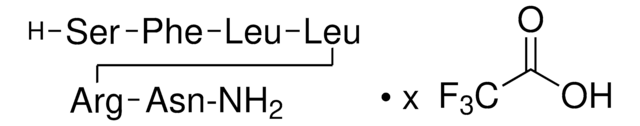539109
PAR-2 Agonist I
The PAR-2 Agonist I controls the biological activity of PAR-2. This small molecule/inhibitor is primarily used for Activators/Inducers applications.
Synonym(s):
PAR-2 Agonist I, Proteinase Activated Receptor-2 Agonist I, 2f-LIGRL-amide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C31H53N9O7
Molecular Weight:
663.81
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
assay
≥95% (HPLC)
form
lyophilized solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
color
white
solubility
DMSO: 5 mg/mL
shipped in
ambient
storage temp.
2-8°C
General description
Native proteinase activated receptor-2- (PAR-2) specific agonist, SLIGRL-NH2, modified with an N-terminal furoyl group to increase its potency and resistance to metabolic degradation. Shown to activate [Ca2+]i mobilization in PAR-2-expressing cells (EC50 = 253 nM in HCT-15 cells) in vitro. Also induces PAR-2-mediated salivation (ED50 = 30 nmol/kg, i.v.) in vivo. Exhibits no effects on PAR-2-/- mice. Reactive towards human, rat, and mouse PAR-2.
The native PAR-2-specific agonist, SLIGRL-NH2, is modified with an N-terminal furoyl group to increase its potency and resistance to metabolic degradation. Shown to activate [Ca2+]i mobilization in PAR-2-expressing cells (EC50 = 253 nM using HCT-15 cells) in vitro and induce PAR-2 mediated salivation (ED50 = 30 nmol/kg, i.v.) in vivo, while exhibiting no effect on PAR-2-/- mice. Reactive towards human, rat, and murine PAR-2.
Biochem/physiol Actions
Cell permeable: no
EC50 = 253 nM as PAR-2-specific agonist using HCT-15 cells
Primary Target
PAR-2
PAR-2
Product does not compete with ATP.
Reversible: no
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Sequence
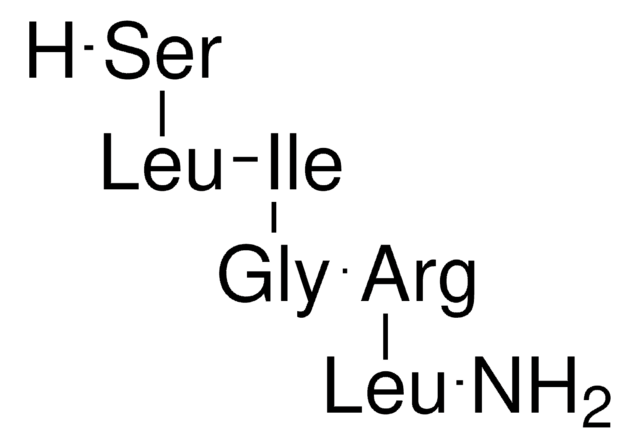
2-Furoyl-Leu-Ile-Gly-Arg-Leu-NH₂
Physical form
Supplied as a trifluoroacetate salt.
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
Other Notes
Kawabata, A., et al. 2004. J. Pharmacol. Exp. Ther.309, 108.
Kawabata, A., et al. 2004. J. Pharmacol. Exp. Ther.309, 1098.
Ferrell, W.R., et al. 2003. J. Clin. Invest.111, 35.
Kawabata, A., et al. 2004. J. Pharmacol. Exp. Ther.309, 1098.
Ferrell, W.R., et al. 2003. J. Clin. Invest.111, 35.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service