681679-M
Iron Chelator IV, 21H7
The Iron Chelator IV, 21H7 controls the biological activity of iron-regulated enzymes. This small molecule/inhibitor is primarily used for Cancer applications.
Synonym(s):
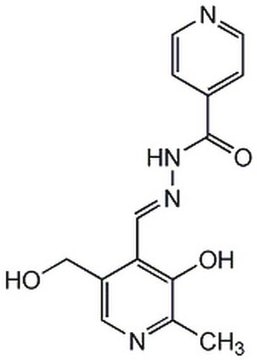
Iron Chelator IV, 21H7, 6-Bromo-Nʹ-(2-hydroxybenzylidene)-2-methylquinoline-4-carbohydrazide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H14BrN3O2
Molecular Weight:
384.23
UNSPSC Code:
12352200
Recommended Products
Quality Level
assay
≥97% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
off-white
solubility
DMSO: 5 mg/mL, light yellow
storage temp.
2-8°C
General description
A cell-permeable salicylaldehyde-acylhydrazone iron chelator that is 20-times more efficient than DFO/desferrioxamine (Cat. No. 252750) in depleting intracellular iron in colon cancer SW480 cells. Iron chelators exert their biological activities by affecting iron-regulated enzymes and singaling events, including HIF1α transcription activation (Effective [21H7] = 10 µM in SW480 and DLD-1 cells) due to inhibition of PHD- (prolyl hydroxylase) mediated HIF1α degradation, as well as stalling and enhancing, respectively, Ferritin and TfRI (Transferrin Receptor I) mRNA translation (Effective [21H7] = 10 µM in SW480 cells) due to iron depletion-induced IRP (Iron Regulatory Protein) IREs (Iron Response Elements) binding. Iron depletion is also reported to inhibit the growths of colorectal adenocarcinoma cultures, DLD-1 (IC50 = 0.6 and 2.9 µM, respectively, by 21H7 and DFO) and SW480 (IC50 = 1.0 and 3.8 µM, respectively, by 21H7 and DFO), as a result of Wnt signaling pathway blockage (Effective conc. = 5 µM 21H7 or 100 µM DFO).
A cell-permeable salicylaldehyde-acylhydrazone iron chelator that is 20-times more efficient than DFO/desferrioxamine (Cat. No. 252750) in depleting intracellular iron in colon cancer SW480 cells. Iron chelators exert their biological activities by affecting iron-regulated enzymes and singaling events, including HIF1α transcription activation due to inhibition of PHD- (prolyl hydroxylase) mediated HIF1α degradation, as well as altered mRNA translations due to enhanced IRP (Iron Regulatory Protein) IREs (Iron Response Elements) binding. Cellular iron depletion is also reported to inhibit the growths of colorectal adenocarcinoma cultures, DLD-1 (IC50 = 0.6 and 2.9 µM, respectively, by 21H7 and DFO) and SW480 (IC50 = 1.0 and 3.8 µM, respectively, by 21H7 and DFO), as a result of Wnt signaling pathway blockage (Effective conc. = 5 µM 21H7 or 100 µM DFO).
Warning
Toxicity: Standard Handling (A)
Preparation Note
Slight warming (45°C) is required for complete solubilization.
Other Notes
Song, S., et al. 2011. Cancer Res.71, 7628.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service