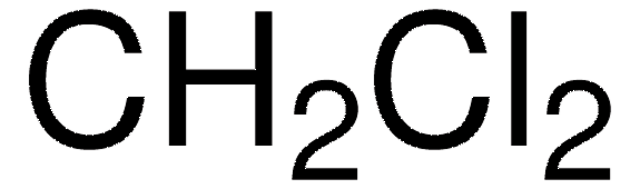8.00549
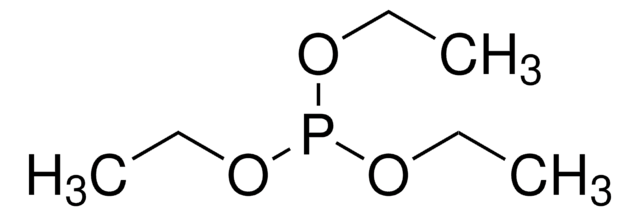
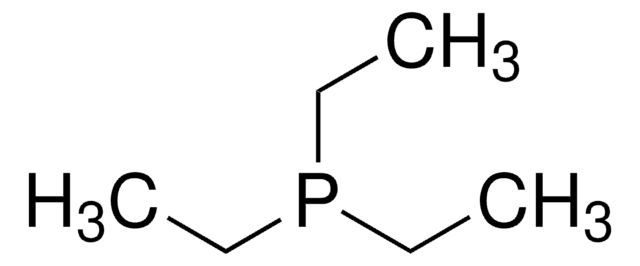
Triethylphosphite
for synthesis
Synonym(s):
Triethylphosphite
About This Item
Recommended Products
vapor pressure
<6 hPa ( 20 °C)
Quality Level
assay
≥97.0% (GC)
form
liquid
autoignition temp.
250 °C
potency
2800 mg/kg LD50, skin (Rabbit)
expl. lim.
3.75-42.5 % (v/v)
bp
156-158 °C/1013 hPa
mp
-112 °C
transition temp
flash point 52 °C
density
0.956 g/cm3 at 20 °C
storage temp.
no temp limit
InChI
1S/C6H15O3P/c1-4-7-10(8-5-2)9-6-3/h4-6H2,1-3H3
InChI key
BDZBKCUKTQZUTL-UHFFFAOYSA-N
Application
- Mechanism of acylative oxidation-reduction-condensation reactions: Research details the use of triethylphosphite as a stoichiometric reductant in acylative oxidation-reduction-condensation reactions, utilizing benzoisothiazolones as oxidants. This study provides insights into the reaction mechanisms and potential applications in synthetic chemistry (Gangireddy et al., 2017).
- Synthesis and antitumor activity of oxazaphosphinane derivatives: A study on the efficient synthesis of novel oxazaphosphinane derivatives using triethylphosphite, exploring their antitumor activities. The research includes X-ray crystallography, DFT studies, and molecular docking to evaluate the potential medical applications (Bahadi et al., 2023).
- Reductive Ireland-Claisen Rearrangements: Triethylphosphite is highlighted in copper-catalyzed reductive Ireland-Claisen rearrangements of propargylic acrylates and allylic allenoates, demonstrating its role in facilitating complex molecular transformations in organic synthesis (Guo et al., 2022).
- Desulfurization under UV light: Triethylphosphite is used in the synthesis of deoxyglycosides through desulfurization processes under UV light, showcasing its utility in glycoscience and the development of new glycoconjugates for various applications (Ge et al., 2017).
- Detection and identification of volatile trialkylphosphites: A derivatization strategy employing triethylphosphite for the detection and identification of volatile trialkylphosphites using liquid chromatography-online solid phase extraction and offline nuclear magnetic resonance spectroscopy, highlighting its analytical applications (Mazumder et al., 2015).
Analysis Note
Density (d 20 °C/ 4 °C): 0.955 - 0.957
Identity (IR): passes test
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Flam. Liq. 3 - Skin Sens. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
129.2 °F - closed cup
flash_point_c
54 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service