8.01949
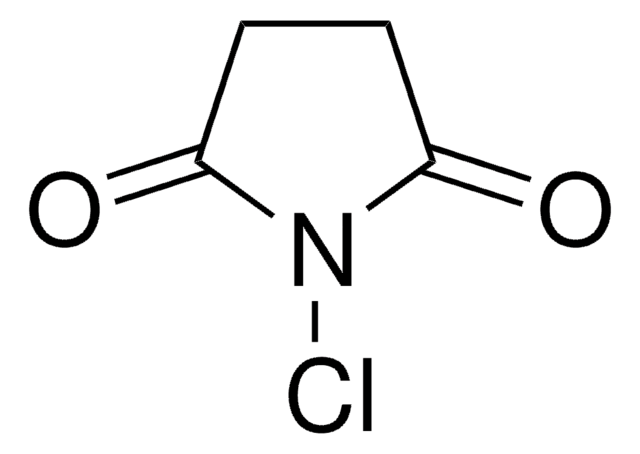
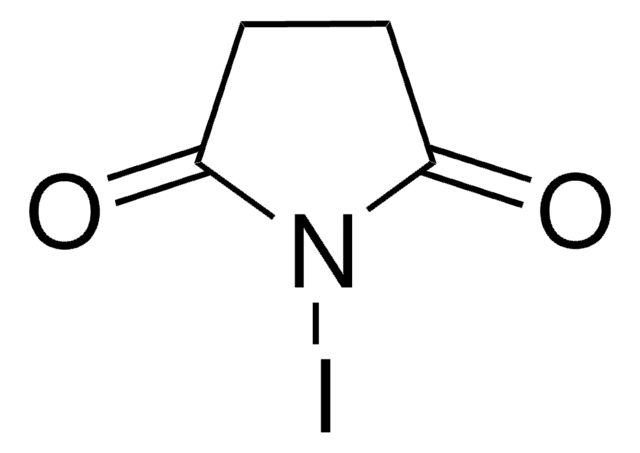
N-Bromosuccinimide
for synthesis
Synonym(s):
N-Bromosuccinimide, NBS
About This Item
Recommended Products
vapor pressure
14.8 hPa ( 20 °C)
Quality Level
form
powder
potency
>2000 mg/kg LD50, oral (Rat)
reaction suitability
reagent type: oxidant
mp
174-179 °C
solubility
14.8 g/L (decomposition)
density
2.1 g/cm3 at 20 °C
bulk density
750 kg/m3
storage temp.
2-8°C
InChI
1S/C4H4BrNO2/c5-6-3(7)1-2-4(6)8/h1-2H2
InChI key
PCLIMKBDDGJMGD-UHFFFAOYSA-N
Related Categories
Application
- Mechanistic study on iodine-catalyzed aromatic bromination of aryl ethers by N-bromosuccinimide: This study explores the roles of NBS in organic synthesis, particularly in radical and electrophilic substitutions (Pramanick et al., 2017).
- One‐Pot Protocol for the Synthesis of Imidazoles and Quinoxalines using N‐Bromosuccinimide: This paper reports a green, efficient synthesis of heterocycles mediated by NBS (Pardeshi et al., 2017).
- N-Bromosuccinimide as an Interfacial Alleviator for Br/I Exchange in Perovskite for Solar Cell Fabrication: This research demonstrates the use of NBS in improving the crystallization and defect passivation in perovskite solar cells (Pegu et al., 2021).
- N‐Bromosuccinimide‐Mediated Radical Cyclization of 3‐Arylallyl Azides: Synthesis of 3‐Substituted Quinolines: Discusses the application of NBS under visible light irradiation to synthesize quinolines (Wang et al., 2015).
- N-Bromosuccinimide mediated synthesis of triazatruxenes from indoles: This study highlights the cyclotrimerization of indoles using NBS, demonstrating its utility as a reagent in complex organic transformations (Toworakajohnkun et al., 2017).
Analysis Note
Identity (IR): passes test
signalword
Warning
Hazard Classifications
Aquatic Acute 1 - Eye Irrit. 2 - Met. Corr. 1 - Muta. 2 - Ox. Sol. 3 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class
5.1B - Oxidizing hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service