8.21111
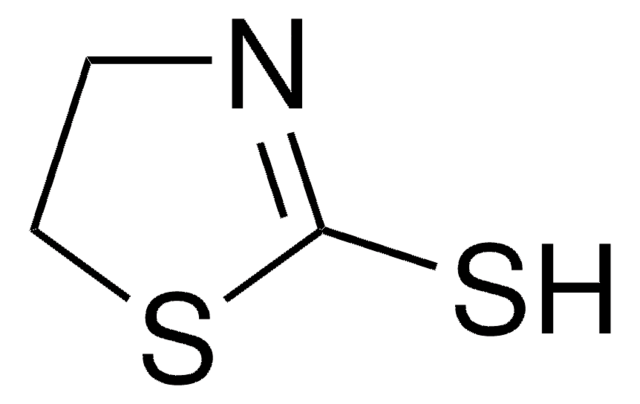
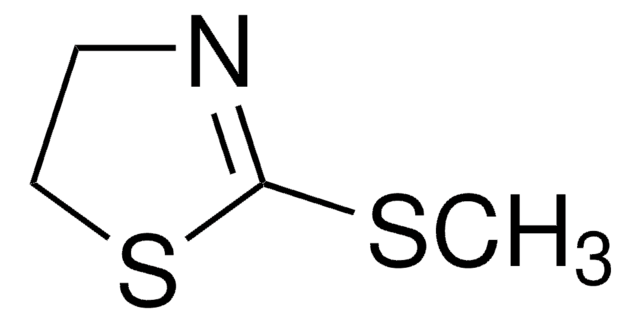
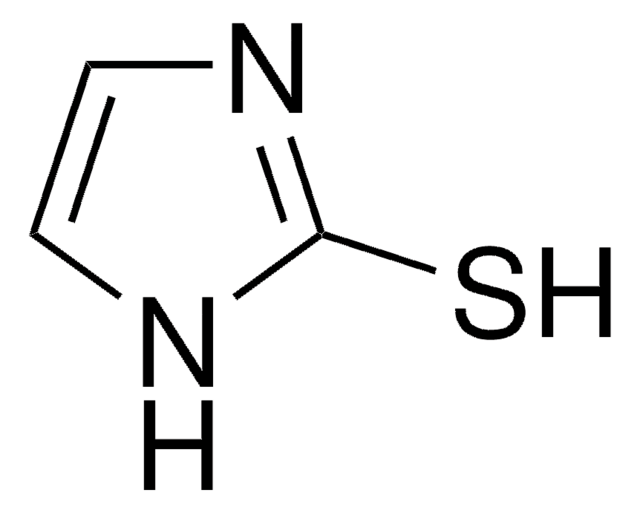
2-Thiazoline-2-thiol
for synthesis
Synonym(s):
2-Thiazoline-2-thiol, 2-Mercaptothiazoline
About This Item
Recommended Products
Quality Level
form
powder
potency
300 mg/kg LD50, oral (Rat)
pH
6-7 (20 °C, 10 g/L in H2O)
mp
101-106 °C
storage temp.
2-30°C
InChI
1S/C3H5NS2/c5-3-4-1-2-6-3/h1-2H2,(H,4,5)
InChI key
WGJCBBASTRWVJL-UHFFFAOYSA-N
Application
- Inducing apoptosis in melanoma cells: A study described a ruthenium(II) complex with 2-mercaptothiazoline ligand inducing selective cytotoxicity by causing DNA damage and apoptosis specifically in melanoma cells, highlighting its potential as a therapeutic agent in oncology (de Melo et al., 2024).
- Antimicrobial and synergistic activities: Thiazoline derivatives, including compounds related to 2-mercaptothiazoline, were studied for their antimicrobial and synergistic effects with conventional antibiotics against multidrug-resistant Staphylococcus aureus, presenting a potential strategy for enhancing antibiotic efficacy (Khan et al., 2020).
- Antimicrobial investigation of metal complexes: The antimicrobial properties of Cd(II) and Sn(II) complexes with 2-mercaptothiazoline were evaluated, underscoring the compound′s role in enhancing the bioactivity of metal-based therapeutics (Gaber et al., 2021).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral
Storage Class
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service