8.51009
PyBOP®
Novabiochem®
Synonym(s):
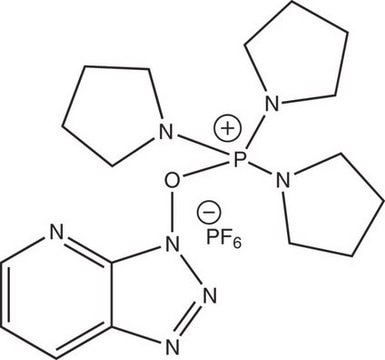
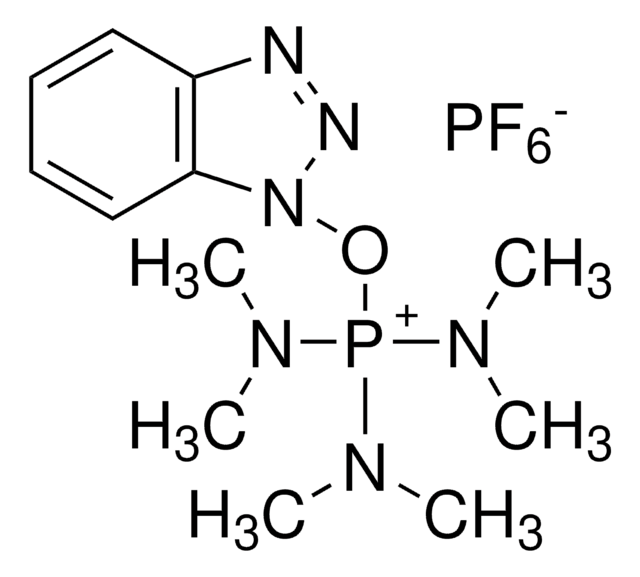
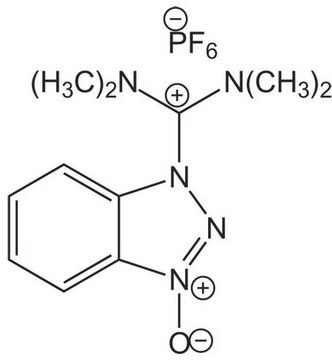
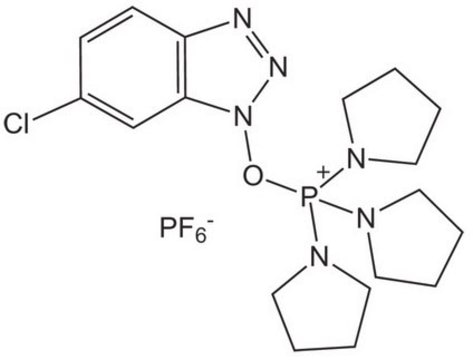
Benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate, PyBOP®
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
assay
≥99.0% (HPLC)
form
powder
reaction suitability
reaction type: Coupling Reactions
manufacturer/tradename
Novabiochem®
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Learn more about the Principles of Green Chemistry.
mp
140-155 °C
application(s)
peptide synthesis
greener alternative category
storage temp.
2-8°C
InChI
1S/C18H28N6OP/c1-2-10-18-17(9-1)19-20-24(18)25-26(21-11-3-4-12-21,22-13-5-6-14-22)23-15-7-8-16-23/h1-2,9-10H,3-8,11-16H2/q+1
InChI key
WGNZRLMOMHJUSP-UHFFFAOYSA-N
Related Categories
General description
Associated Protocols and Technical Articles
Guide to Selection of Coupling Reagents
Literature references
[1] J. Martinez, et al. (1985) J. Med. Chem., 28, 1874.
[2] J. Coste, et al. (1990) Tetrahedron Lett., 31, 205.
[3] G. B. Fields, et al. in ′Innovation & Perspectives in Solid Phase Synthesis, 1st International Symposium′, R. Epton (Eds), SPCC UK Ltd., Birmingham, 1990, pp. 241.
[4] T. Høeg-Jensen, et al. (1991) Tetrahedron Lett., 32, 7617.
[5] R. von Eggelkraut-Gottanka, et al. (2003) Tetrahedron Lett., 44, 3551.
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 99.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Legal Information
Still not finding the right product?
Give our Product Selector Tool a try.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Novabiochem® offers a large number of coupling reagents for in situ activation. In situ activating reagents are easy to use, fast reacting – even with sterically hindered amino acids, and their use is generally free of side reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service