AB9658
Anti-Tau phospho Serine 396 Antibody
Chemicon®, from rabbit
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
rabbit
Quality Level
antibody form
affinity purified immunoglobulin
antibody product type
primary antibodies
clone
polyclonal
purified by
affinity chromatography
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
western blot: suitable
NCBI accession no.
UniProt accession no.
shipped in
dry ice
target post-translational modification
phosphorylation (pSer396)
Gene Information
human ... MAPT(4137)
General description
Tau is a neuronal microtubule-associated protein found predominantly on axons and functions to promote tubulin polymerization and stabilize microtubules. Tau, in its hyperphosphorylated form, is the major component of paired helical filaments (PHF), the building block of neurofibrillary lesions in Alzheimer′s disease (AD) brain. Hyperphosphorylated Tau is also found in neurofibrillary lesions in a range of other central nervous system disorders. Hyperphosphorylation impairs the microtubule binding function of Tau, resulting in the destabilization of microtubules in AD brains, ultimately leading to the degeneration of the affected neurons. Numerous serine/threonine kinases, including GSK-3beta, protein kinase A (PKA), cyclin-dependent kinase 5 (cdk5) and casein kinase II (CK2), phosphorylate Tau. Serine 396 is phosphorylated by GSK-3beta and cdk5 in vitro and in vivo.
Specificity
Tau phosphoSerine 396. The antibody recognizes Tau pSerine 396 in samples of recombinant human Tau treated with GSK-3beta for 45 minutes. The reactivity of the antibody is blocked with the pSerine 396 peptide but not the non-phosphopeptide or a generic phosphoSerine-containing peptide.
The immunogen is conserved in rat, mouse, rhesus monkey, goat, bovine and baboon.
Immunogen
Synthetic peptide of amino acids surrounding the phosphoSerine 396 site of human Tau.
Application
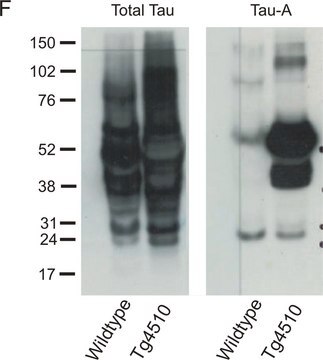
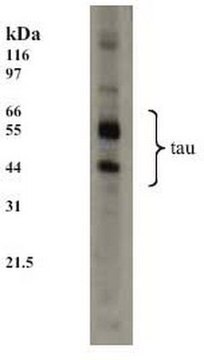
Anti-Tau phospho Serine 396 Antibody detects level of Tau phospho Serine 396 & has been published & validated for use in WB.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
wgk_germany
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protein kinase C and calcium / calmodulin-dependent protein kinase II phosphorylate three-repeat and four-repeat tau isoforms at different rates.
Singh, T.J., et al.
Molecular and Cellular Biochemistry, 168(1-2), 141-148 (1997)
E Sontag et al.
Neuron, 17(6), 1201-1207 (1996-12-01)
Recently, we reported that a pool of protein phosphatase 2A (PP2A) is associated with microtubules. Here, we demonstrate that specific isoforms of PP2A bind and dephosphorylate the neuronal microtubule-associated protein tau. Coexpression of tau and SV40 small t, a specific
G A Jicha et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 19(17), 7486-7494 (1999-08-25)
To elucidate the role cAMP-dependent protein kinase (PKA) phosphorylations on tau play in Alzheimer's disease, we have generated highly specific monoclonal antibodies, CP-3 and PG-5, which recognize the PKA-dependent phosphorylations of ser214 and ser409 in tau respectively. The present study
Jean C Augustinack et al.
Acta neuropathologica, 103(1), 26-35 (2002-02-12)
Microtubule associated protein tau is abnormally phosphorylated in Alzheimer's disease (AD) and aggregates as paired helical filaments (PHFs) in neurofibrillary tangles (NFTs). We show here that the pattern of tau phosphorylation correlates with the loss of neuronal integrity. Studies using
A D Alonso et al.
The Journal of biological chemistry, 276(41), 37967-37973 (2001-08-10)
The microtubule-associated protein tau is a family of six isoforms that becomes abnormally hyperphosphorylated and accumulates in neurons undergoing neurodegeneration in the brains of patients with Alzheimer disease (AD). We investigated the isoform-specific interaction of normal tau with AD hyperphosphorylated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service