CC1034
ADAMTS-5, recombinant human truncated
Synonym(s):
Aggrecanase 2
About This Item
Recommended Products
biological source
human
Quality Level
form
liquid
manufacturer/tradename
Chemicon®
concentration
>50% (truncated ADAMTS-5 of total protein of the preparation, SDS-PAGE)
0.1 mg/mL (total protein concentration)
0.1 mg/mL
NCBI accession no.
UniProt accession no.
shipped in
dry ice
Gene Information
human ... ADAMTS5(11096)
General description
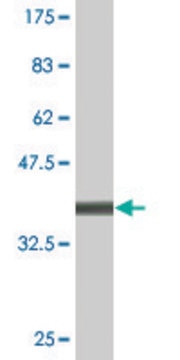
Molecular form: Recombinant human ADAMTS-5 D625-930 is produced with the baculo-virus expression system and purified from insect cell culture supernatants. The protein contains the catalytic domain, the disintegrin domain and the thrombospondin type-1 motif of full-length ADAMTS-5 followed by a C-terminal His6-tag. The calculated Mr of the amino acid sequence is 40.8 kDa. In SDS-PAGE the protein exhibits a Mr of about 50 kDa.
ACTIVITY:
Aggrecanase activity of the ADAMTS-5 preparation is determined with recombinant aggrecan interglobular domain (Aggrecan-IGD from Chemicon). ADAMTS-5 hydrolyzes the "aggrecanase" site within this domain (peptide bond E373 -A374 in human aggrecan). The recombinant substrate is incubated at a concentration of 0.1 μM with ADAMTS-5 in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM CaCl2, 1 μM leupeptin, 1 μM pepstatin, 1 mM Pefabloc, 0.05 % Brij 35 for 15 min at 37 °C. Substrate cleavage at the "aggrecanase"-site is estimated from the appearance of the hydrolysis fragment with the novel N-terminus ARGSVIL. The fragment is quantified with two monoclonal antibodies. Under the specified conditions the hydrolysis rate is >0.5 nmoles hydrolyzed substrate/ min . ml ADAMTS-5 preparation or >5 nmoles hydrolyzed substrate/ min . mg.
Inhibitors: ADAMTS-5 is inhibited by tissue inhibitor of matrix metalloproteinase 3 (TIMP3) and by alpha2-macroglobulin. Enzyme activity is also suppressed by chelators of divalent cations such as EDTA and by synthetic metalloproteinase inhibitors.
Application
Degradation of extracellular matrix proteoglycans
Screening and characterisation of inhibitors
Standard in enzymatic and immunological assays
Optimal working dilutions must be determined by the end user.
Physical form
Storage and Stability
Legal Information
Disclaimer
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1D - Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service