ECM557
QCM Leukocyte Transendothelial Migration Assay (Colorimetric, 24 Assays)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352207
eCl@ss:
32161000
NACRES:
NA.32
Recommended Products
manufacturer/tradename
Chemicon®
QCM
Quality Level
technique(s)
cell based assay: suitable
detection method
colorimetric
General description
Leukocytes patrol the vascular system. They must migrate across endothelial barriers in order to recruit to sites of inflammation. This process involves a multistep cascade consisting of leukocyte rolling, adhesion, and transmigration. A quantitative assay for leukocyte transendothelial migration has been described using a modified Boyden chamber system (Roth et al. 1995, Ding et al. 2000). The Boyden chamber system is a two chamber system with a porous membrane providing an interface between these two chambers. Endothelial cells are cultured on top of the porous membrane that is coated with an extracelluar matrix (ECM) protein. Once a confluent layer of cells is established, leukocytes are then added onto the endothelial monolayer. Leukocyte migration across the endothelium is determined by measuring the number of cells that migrate between the endothelial cells, through the porous membrane, to the lower chamber.
Millipore′s Colorimetric QCM Leukocyte Transendothelial Cell Migration Assay provides an efficient model to analyze the ability of leukocytes to migrate through the endothelium. The assay is designed with a 3 μm pore size cell culture insert, appropriate for most leukocyte migration. The upper side of the cell culture insert is coated with fibronectin to support the optimal attachment and growth of endothelial cells. The assay allows investigators to evaluate the effects of various factors that influence the transendothelial process.
Precoated cell culture inserts are provided in the Millipore QCM Leukocyte Transendothelial Cell Migration Assay to significantly reduce assay time. Additionally, the assay allows quantitative analysis of leukocyte migration. Following incubation of leukocytes with the endothelial cell layer, migratory cells are stained and quantified with WST-1 reagent and measured using a spectrophotometer. Absorbance correlates with cell migration.
Precoated cell culture inserts are provided in the Millipore QCM Leukocyte Transendothelial Cell Migration Assay to significantly reduce assay time. Additionally, the assay allows quantitative analysis of leukocyte migration. Following incubation of leukocytes with the endothelial cell layer, migratory cells are stained and quantified with WST-1 reagent and measured using a spectrophotometer. Absorbance correlates with cell migration.
Application
Research Category
Cell Structure
Cell Structure
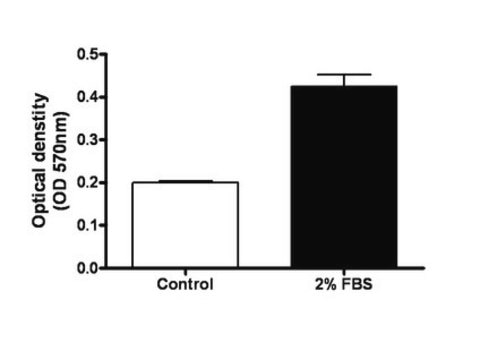
Results of the assay may be illustrated graphically using a bar chart to display optical density (OD) values at 420 - 480 nm.
Additional migration may also be induced or inhibited in test wells through the addition of cytokines or other pharmacological agents.
Figures 2 and 3 demonstrate typical migration results. One should use the data for reference only. This data should not be used to interpret actual assay results.
Additional migration may also be induced or inhibited in test wells through the addition of cytokines or other pharmacological agents.
Figures 2 and 3 demonstrate typical migration results. One should use the data for reference only. This data should not be used to interpret actual assay results.
This Colorimetric QCM Leukocyte Transendothelial Cell Migration Assay provides an efficient model to analyze the ability of leukocytes to migrate through the endothelium.
Packaging
24 Assays
Components
1. Migration Test Plate: (Part No. CS202626) Two 24-well culture plates, each containing 12 human fibronectin-coated cell culture inserts (3 μm pore size), sufficient for the evaluation of 24 test samples
2. TNFα: (Part No. 2004167) One tube - 20 μL at 0.1 mg/mL
3. Cell Stain Solution: (Part No. 20294) One vial - 10 mL
4. WST-1 Reagent: (Part No. CS201370) One vial.
5. Electro Coupling Solution: (Part No. CS201382) One vial - 5 mL.
6. 96 well Stain Quantitation Plate: (Part No. 2005870) One plate.
7. Forceps: (Part No. 10203) 1 pair
2. TNFα: (Part No. 2004167) One tube - 20 μL at 0.1 mg/mL
3. Cell Stain Solution: (Part No. 20294) One vial - 10 mL
4. WST-1 Reagent: (Part No. CS201370) One vial.
5. Electro Coupling Solution: (Part No. CS201382) One vial - 5 mL.
6. 96 well Stain Quantitation Plate: (Part No. 2005870) One plate.
7. Forceps: (Part No. 10203) 1 pair
Storage and Stability
The kit is packaged in two individual boxes. ECM557-1 contains components that upon arrival should be stored at 2° to 8°C. ECM557-2 contains TNFα , WST-1, and Electro Coupling Solution that upon arrival should be stored at -20°C. Please refer to k
Legal Information
Accutase is a registered trademark of Innovative Cell Technologies, Inc.
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial
wgk_germany
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Cell based angiogenesis assays to analyze new blood vessel formation for applications of cancer research, tissue regeneration and vascular biology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service