EI8
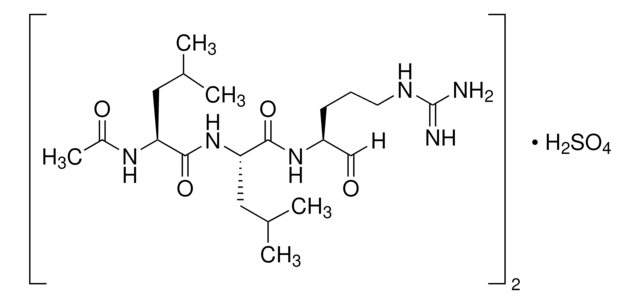
Leupeptin
Synonym(s):
N-Acetyl-L-leucyl-L-leucyl-L-argininal, Ac-Leu-Leu-Arg-H, Acetyl-L-leucyl-L-leucylargininal, Leupeptin hemisulfate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352200
eCl@ss:
32160405
NACRES:
NA.77
Recommended Products
General description
Leupeptin is a water-soluble and cell-permeable organic compound. It is produced by various species of actinomycetes and several other fungal families.
Application
Leupeptin has been used as a protease inhibitor supplement in cell lysis buffer for sample preparation.
Biochem/physiol Actions
Leupeptin serves as a lysosomal protease and calpain (serine- and cysteine-like protease) inhibitor. It may be used to reduce the cell death induced by excess calpain activation. Leupeptin confers significant protection against hair cell damage caused by gentamicin ototoxicity. In addition, it also impedes protein degradation in denervated rat muscles and muscles of mice with hereditary muscular dystrophy. Thus, leupeptin may be beneficial in hindering tissue atrophy.
Physical form
Lyophilized.
Storage and Stability
Maintain dry at -20ºC for up to 18 months after date of receipt. Store reconstituted product in aliquots at -20ºC for up to 6 months. Avoid repeated thaw freeze cycles.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The structure and activity of leupeptins and related analogs.
K Maeda et al.
The Journal of antibiotics, 24(6), 402-404 (1971-06-01)
P Libby et al.
Science (New York, N.Y.), 199(4328), 534-536 (1978-02-03)
The protease inhibitor leupeptin decreases protein degradation in rat skeletal and cardiac muscle incubated in vitro, while protein synthesis remains unaltered. Leupeptin also lowers protein breakdown in denervated rat muscles and affected muscles from mice with hereditary muscular dystrophy. Leupeptin
Erika Billinger et al.
FEBS open bio, 10(12), 2605-2615 (2020-10-06)
Leupeptin is a naturally occurring inhibitor of various proteases, in particular serine proteases. Following its discovery, the inhibitory properties of several other peptidyl argininals have been studied. The specificity of leupeptin is most likely due to the Leu-Leu-Argininal sequence, and
Christian Windpassinger et al.
American journal of human genetics, 101(3), 391-403 (2017-09-09)
In five separate families, we identified nine individuals affected by a previously unidentified syndrome characterized by growth retardation, spine malformation, facial dysmorphisms, and developmental delays. Using homozygosity mapping, array CGH, and exome sequencing, we uncovered bi-allelic loss-of-function CDK10 mutations segregating
Biological activities of leupeptins.
T Aoyagi et al.
The Journal of antibiotics, 22(11), 558-568 (1969-11-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service