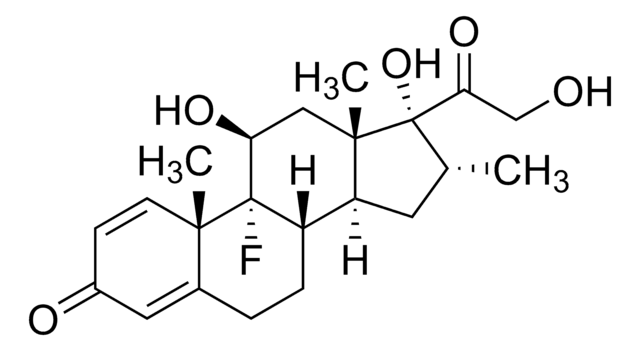
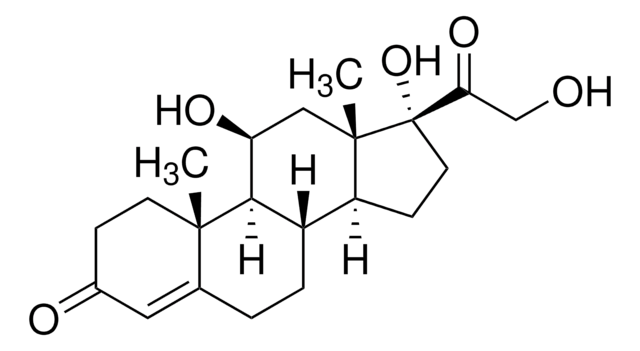
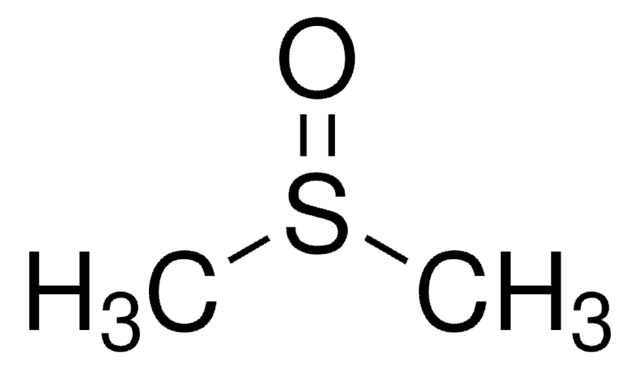
IM09L
Anti-MMP-9 (Ab-1) Mouse mAb (6-6B)
lyophilized, clone 6-6B, Calbiochem®
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified antibody
antibody product type
primary antibodies
clone
6-6B, monoclonal
form
lyophilized
does not contain
preservative
species reactivity
human
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
isotype
IgG1
shipped in
ambient
storage temp.
2-8°C
target post-translational modification
unmodified
Gene Information
human ... MMP9(4318)
General description
Matrix metalloproteinases (MMP′s) are a family of enzymes that are responsible for the degradation of extracellular matrix components such as collagen, laminin and proteoglycans. In addition to sequence homology, all MMP′s share the following characteristics: the catalytic mechanism is dependent upon a zinc ion at the active center, they cleave one or more extracellular matrix components, they are secreted as zymogens which are activated by removal of an ~10 kDa segment from the N terminus and they are inhibited by tissue inhibitor of metalloproteinases (TIMP). These enzymes are involved in normal physiological processes such as embryogenesis and tissue remodeling and may play an important role in arthritis, periodontitis, and metastasis.MMP-9 (Gelatinase B, 92 kDa gelatinase/type IV collagenase) is secreted as a 92 kDa zymogen which is proteolytically processed to the 83 kDa active form; a 68 kDa has also been detected. MMP-9, along with its most closely related member of the MMP family, MMP-2, show substrate specificity toward type IV and V collagens, gelatin and elastin. Numerous studies have shown a correlation between collagenase expression and metastatic potential. Elevated levels of MMP-9 in plasma suggest that it may be a useful marker for the diagnosis or prognosis of cancer in general.
Purified mouse monoclonal antibody. Recognizes the ~92 kDa latent and the ~83 kDa active forms of human MMP-9 under non-reducing conditions, but only the latent form under reducing conditions.
Recognizes the ~92 kDa latent and the ~83 kDa active forms of MMP-9 under non-reducing conditions, but only the latent form under reducing conditions. Inhibits the enzymatic activity of MMP-9. For paraffin sections, use Cat. No. IM37L.
This Anti-MMP-9 (Ab-1) Mouse mAb (6-6B) is validated for use in Immunoblotting, Immunoprecipitation, Neutralization Studies, Paraffin Sections for the detection of MMP-9 (Ab-1).
Immunogen
Human
MMP-9 from conditioned medium of PMA-stimulated HT-1080 human fibrosarcoma cells
Application
Immunoblotting (2 µg/ml)
Immunoprecipitation (see appplication references)
Neutralization Studies (see comments and application references)
Paraffin Sections (not recommended)
Immunoprecipitation (see appplication references)
Neutralization Studies (see comments and application references)
Paraffin Sections (not recommended)
Warning
Toxicity: Standard Handling (A)
Physical form
Lyophilized from a volatile buffer, 100 µg BSA.
Reconstitution
Store at 4°C until reconstituted, then store in aliquots at -20°C or at 4°C with 0.1% azide. Resuspend the antibody with sterile PBS, pH 7.4, or sterile 20 mM Tris-saline, pH 7.4, to yield a final concentration of 100 µg/ml; product will be more stable if 0.1% sodium azide is included (do not add azide if antibody is to be used with viable cells). Lyophilized antibodies should be resuspended at 4°C with occasional gentle mixing for at least 2 h. Freezing of aliquots is best for long-term storage of reconstituted product; repetitive freezing and thawing should be avoided.
Analysis Note
Positive Control
MMP-9 protein (Cat. Nos. PF024 or PF038)
MMP-9 protein (Cat. Nos. PF024 or PF038)
Other Notes
Cottam, D.W. and Rees, R.C. 1993. Intl. J. of Oncol.2, 861.
Nakajima, M., et al. 1993. Cancer Res.53, 5802.
Ramos-DeSimone, et al. 1993. Hybridoma. 12, 349.
Stetler-Stevenson, W.G., et al. 1993. FASEB.7, 1434.
Zucker, S., et al. 1993. Cancer Res.53, 140.
Woessner, J.F. 1991. FASEB. 5, 2145.
Liotta, L.A. and Stetler-Stevenson, W.G. 1990. in Seminars in Cancer Biology, ed. M.M. Gottesman. Vol. 1; 99-106.
Nakajima, M., et al. 1993. Cancer Res.53, 5802.
Ramos-DeSimone, et al. 1993. Hybridoma. 12, 349.
Stetler-Stevenson, W.G., et al. 1993. FASEB.7, 1434.
Zucker, S., et al. 1993. Cancer Res.53, 140.
Woessner, J.F. 1991. FASEB. 5, 2145.
Liotta, L.A. and Stetler-Stevenson, W.G. 1990. in Seminars in Cancer Biology, ed. M.M. Gottesman. Vol. 1; 99-106.
Inhibits MMP-9 enzymatic activity. For staining paraffin sections, use Anti-MMP-9 (626-644) (Ab-3) Mouse mAb (56-2A4) (Cat. No. IM37L). Antibody should be titrated for optimal results in individual systems.
Legal Information
Not available for sale in Japan.
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
wgk_germany
WGK 2
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yao Tong et al.
Journal of inflammation research, 14, 5079-5094 (2021-10-23)
Acute lung injury (ALI) is a severe respiratory disease with high rates of morbidity and mortality. Many mediators regarding endogenous or exogenous are involved in the pathophysiology of ALI. Here, we have uncovered the involvement of integrins and matrix metalloproteinases
Min Hee Park et al.
Stem cells (Dayton, Ohio), 34(8), 2145-2156 (2016-04-20)
Hematopoietic stem/progenitor cell (HSPC) mobilization is an essential homeostatic process regulated by the interaction of cellular and molecular components in bone marrow niches. It has been shown by others that neurotransmitters released from the sympathetic nervous system regulate HSPC egress
Daniel J Price et al.
Cell communication & adhesion, 9(2), 87-102 (2002-12-19)
We studied the invasion of HMT-3522 breast epithelial cells in response to epidermal growth factor (EGF), and the associated signaling pathways. HMT-3522 T4-2 cells were shown to invade Matrigel-coated Transwell membranes in response to EGF while HMT-3522 S-1 cells failed
Andreas Behren et al.
Cancer research, 65(24), 11613-11621 (2005-12-17)
Papillomaviruses are involved in the development of cancers of the female cervix, head and neck, and skin. An excellent model to study papillomavirus-induced tumor induction and progression is the New Zealand White rabbit, where the skin is infected with the
Agnes Schäfer et al.
Frontiers in oncology, 12, 826273-826273 (2022-04-05)
Glioblastoma (GBM) as the most common and aggressive brain tumor is characterized by genetic heterogeneity, invasiveness, radio-/chemoresistance, and occurrence of GBM stem-like cells. The metalloprotease-disintegrin ADAM8 is highly expressed in GBM tumor and immune cells and correlates with poor survival.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service