MAB351
Anti-Glutamate Decarboxylase Antibody, 65 kDa isoform, clone GAD-6
clone GAD-6, Chemicon®, from mouse
Synonym(s):
GAD65
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
GAD-6, monoclonal
species reactivity
rat, human
manufacturer/tradename
Chemicon®
technique(s)
immunohistochemistry: suitable
western blot: suitable
isotype
IgG2a
NCBI accession no.
UniProt accession no.
shipped in
dry ice
target post-translational modification
unmodified
Gene Information
human ... GAD2(2572)
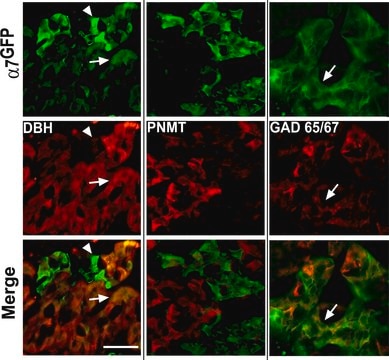
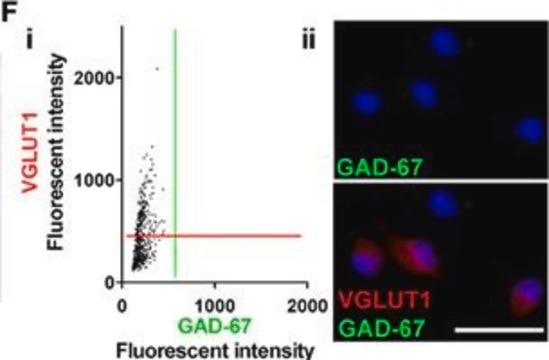
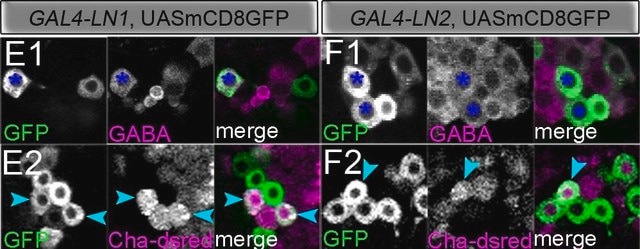
General description
Specificity
Immunogen
Application
Western blot
Optimal working dilutions must be determined by end user.
APPLICATION NOTES FOR MAB351
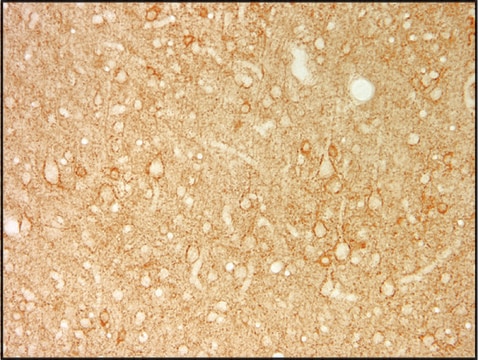
IMMUNOHISTOCHEMISTRY
1) Perfuse rats with 100 mM phosphate buffer, pH 7.4 containing 1% paraformaldehyde, 0.34% L-lysine and 0.05% m-periodate (1% PLP).
2) Postfix brains in 1% PLP for 1-2 hours.
3) Transfer brains to 100 mM phosphate buffer containing 30% sucrose and gently agitate on a shaker platform at +4°C for 48-60 hours.
4) Using a sliding microtome, cut 30 mm sections of frozen cerebellum. As the sections are cut, collect them in a vial of cold 100 mM phosphate buffer.
5) Incubate sections in PBS containing 1.5% normal serum and 0.2% Triton X-100 for 30 minutes.
6) On a shaker platform, incubate sections with MAB351 (diluted 1 μg/mL in PBS containing 1.5% normal serum and 0.2% Triton X-100) for 12-36 hours at +4°C.
7) On a shaker platform, rinse sections eight times, 10-15 minutes per rinse, in PBS.
8) Detect with standard secondary antibody detection system (PAP, ABC, etc.).
9) Mount sections, dehydrate, and apply coverslips.
Neuroscience
Neurotransmitters & Receptors
Target description
Physical form
Storage and Stability
Analysis Note
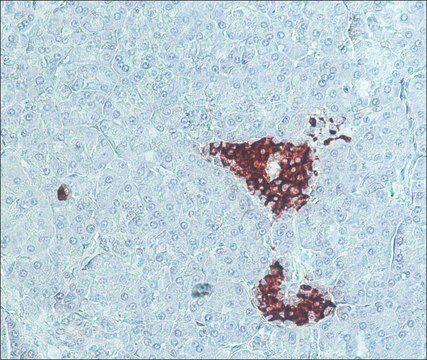
Rat brain tissue
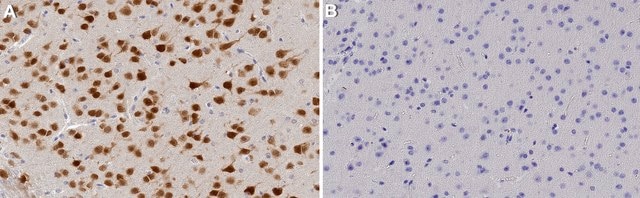
human brain lysate
Other Notes
Legal Information
Disclaimer
Still not finding the right product?
Give our Product Selector Tool a try.
recommended
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service