MAB8121
Anti-Cytomegalovirus Antibody, blend, clone 8B1.2, 1G5.2, and 2D4.2
Chemicon®, from mouse
Synonym(s):
CMV
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
1G5.2, monoclonal
2D4.2, monoclonal
8B1.2, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
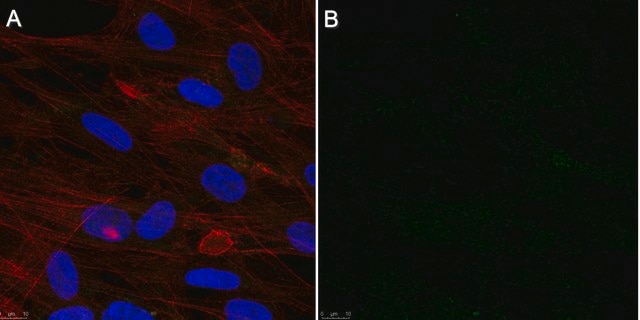
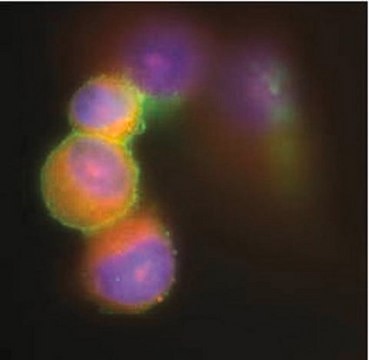
immunofluorescence: suitable
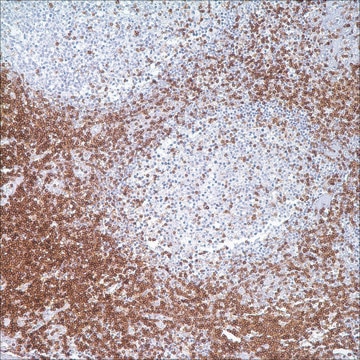
immunohistochemistry: suitable
isotype
IgG2a
shipped in
wet ice
Specificity
Reacts with Immediate early, early, and late antigen preparations. Can detect CMV infection within 2 hours post-infection exhibiting a nuclear staining which reaches peak intensity between 48 and 96 hours. This antigen is persisting and can be detected during the complete CMV infection cycle. Staining of CMV structural antigens becomes apparent at about 48 hours. Extra-nuclear staining is first seen in the region of the golgi apparatus followed subsequently by staining of viral proteins and particles in the cytoplasm.
With CMV the antigens expressed at different times are listed as:
Immediate Early (alpha gene expression): those antigens expressed at 3-12 hrs post-infection generally involved in Transcription such as 72kD major phosphoprotein and a few other other antigens at 60 - 80kD.
Early Antigen (beta genes) a.k.a. Delayed Early or Intermediate Early: expressed at 12-24hrs post-infection. Generally enzymes and one virion structural gene preceding viral DNA synthesis.
Late Antigen (gamma genes): expressed at 36-48hrs post-infection. Generally structural proteins. Major protein = 55kD
With CMV the antigens expressed at different times are listed as:
Immediate Early (alpha gene expression): those antigens expressed at 3-12 hrs post-infection generally involved in Transcription such as 72kD major phosphoprotein and a few other other antigens at 60 - 80kD.
Early Antigen (beta genes) a.k.a. Delayed Early or Intermediate Early: expressed at 12-24hrs post-infection. Generally enzymes and one virion structural gene preceding viral DNA synthesis.
Late Antigen (gamma genes): expressed at 36-48hrs post-infection. Generally structural proteins. Major protein = 55kD
Immunogen
Epitope: blend
MRC-5 infected cells with ATCC VR538 and AD169, ultrasonicated, screened by plate reduction neutralization tests.
Application
Immunohistochemistry
Immunofluorescence
Optimal working dilutions must be determined by the end user.
Immunofluorescence
Optimal working dilutions must be determined by the end user.
This Anti-Cytomegalovirus Antibody, blend, clone 8B1.2, 1G5.2 & 2D4.2 is validated for use in IF, IH for the detection of Cytomegalovirus.
Physical form
0.02 M PB, 0.25M NaCl, PH 7.6 with 0.1% Sodium Azide.
Format: Purified
Storage and Stability
Maintain at +2-8C in convenient aliquots for up to 6 months. Do not freeze.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
recommended
Product No.
Description
Pricing
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Intrauterine growth restriction caused by underlying congenital cytomegalovirus infection.
Pereira, L; Petitt, M; Fong, A; Tsuge, M; Tabata, T; Fang-Hoover, J; Maidji, E; Zydek et al.
The Journal of Infectious Diseases null
Takako Tabata et al.
Journal of virology, 89(9), 5134-5147 (2015-03-06)
Human cytomegalovirus (HCMV) is a major cause of birth defects that include severe neurological deficits, hearing and vision loss, and intrauterine growth restriction. Viral infection of the placenta leads to development of avascular villi, edema, and hypoxia associated with symptomatic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service