MABE302
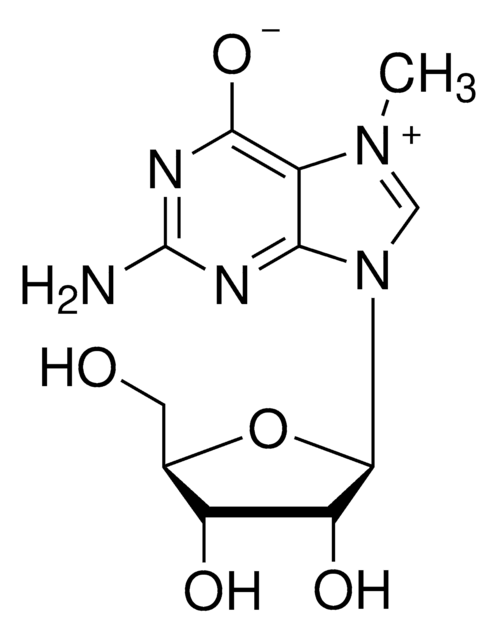
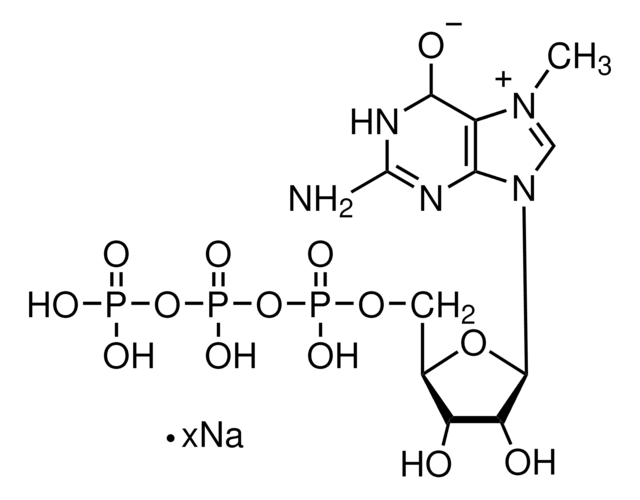
Anti-2,2,7-Trimethylguanosine Antibody, clone K121
clone K121, from mouse
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
K121, monoclonal
species reactivity
human, mouse
technique(s)
affinity chromatography: suitable
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
isotype
IgG1κ
shipped in
wet ice
target post-translational modification
unmodified
General description
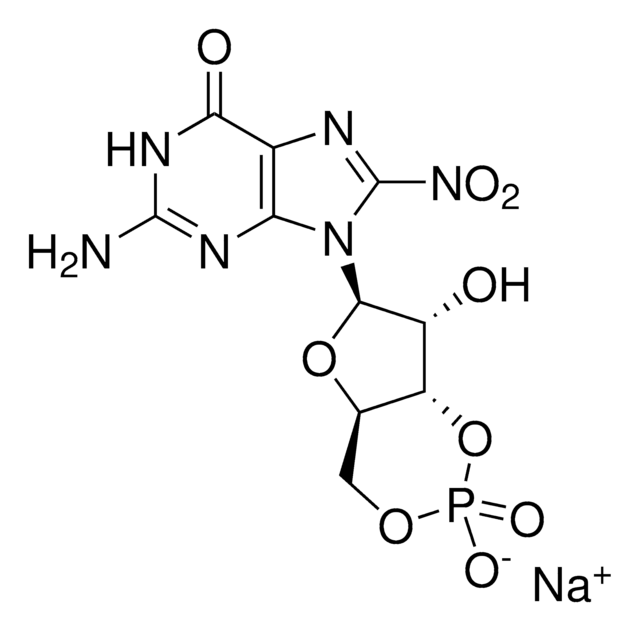
The 2,2,7-trimethylguanosine cap structure is found on the 5’ end of small nuclear RNAs and small nucleolar RNAs. Initially, these RNA molecules are capped with 7-monomethylguanosine (m7G) which is then methylated by the trimethylguanosine synthase enzyme to form 2,2,7-trimethylguanosine. The synthesis of 2,2,7-trimethylguanosine occurs in the cytoplasm and is an important marker for nuclear localization of RNA molecules. Capping of RNAs is an important requirement for RNA transport and splicing processes. Uncapped RNAs are rapidly degraded by the 5′ exoribonuclease enzyme. 2,2,7-trimethylguanosine capping may play a role in the expression of viral RNAs.
Application
Anti-2, 2, 7-Trimethylguanosine Antibody, clone K121 is a high quality Mouse Monoclonal Antibody for the detection of 2, 2, 7-Trimethylguanosine & has been validated in ICC, PAI & IP.
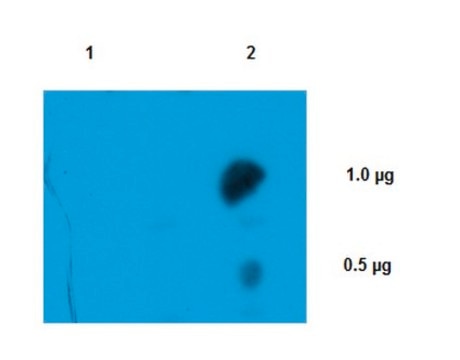
Immunoaffinity Purification Analysis: A representative lot from an independent laboratory was used in IAP (Krainer, A.R., et al. (1988). Nucleic Acids Res. 16(20):9415-9429.).
Immunoprecipitation Analysis: A representative lot from an independent laboratory was immunoprecipitated in IP (Moketi, S., et al. (2002). Mol Cell. 10(3):599-609.).
Immunoprecipitation Analysis: A representative lot from an independent laboratory was immunoprecipitated in IP (Moketi, S., et al. (2002). Mol Cell. 10(3):599-609.).
Quality
Evaluated by Immunocytochemistry in NIH/3T3, HeLa, and A431 cells.
Immunocytochemistry Analysis: A 1:500 dilution of this antibody detected 2,2,7-trimethylguanosine in NIH/3T3, HeLa, and A431 cells.
Immunocytochemistry Analysis: A 1:500 dilution of this antibody detected 2,2,7-trimethylguanosine in NIH/3T3, HeLa, and A431 cells.
Physical form
Format: Purified
Analysis Note
Control
NIH/3T3, HeLa, and A431 cells
NIH/3T3, HeLa, and A431 cells
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Not finding the right product?
Try our Product Selector Tool.
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cyrille Girard et al.
Nucleic acids research, 34(10), 2925-2932 (2006-06-02)
Neuronal degeneration in spinal muscular atrophy (SMA) is caused by reduced expression of the survival of motor neuron (SMN) protein. The SMN protein is ubiquitously expressed and is present both in the cytoplasm and in the nucleus where it localizes
Magdalena Strzelecka et al.
Nucleus (Austin, Tex.), 1(1), 96-108 (2011-02-18)
The Cajal body (CB) is an evolutionarily conserved nuclear subcompartment, enriched in components of the RNA processing machinery. The composition and dynamics of CBs in cells of living organisms is not well understood. Here we establish the zebrafish embryo as
Pre-mRNA splicing by complementation with purified human U1, U2, U4/U6 and U5 snRNPs.
Krainer, A R
Nucleic Acids Research, 16, 9415-9429 (1988)
Cristina Moreno-Castro et al.
Journal of cell science, 132(22) (2019-10-23)
Cajal bodies are nuclear organelles involved in the nuclear phase of small nuclear ribonucleoprotein (snRNP) biogenesis. In this study, we identified the splicing factor TCERG1 as a coilin-associated factor that is essential for Cajal body integrity. Knockdown of TCERG1 disrupts
Masaki Kobayashi et al.
Disease models & mechanisms, 10(3), 215-224 (2017-03-03)
Unique deficits in the function of adult sensory neurons as part of their early neurodegeneration might account for progressive polyneuropathy during chronic diabetes mellitus. Here, we provide structural and functional evidence for aberrant pre-mRNA splicing in a chronic type 1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service