MCE50027H1
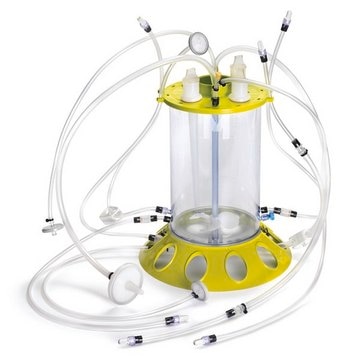
Millistak+® Pod Depth Filter
CE50, cellulose, filtration area 0.027 m2, hydrophilic
Synonym(s):
Millistak+ Pod Depth Filter, CE50 media series, 0.027 m2 surface area, 1/4 in. Hose Barb connection
About This Item
Recommended Products
material
cellulose
glass fiber-reinforced polypropylene
Quality Level
description
For clarification of serum, plasma, vaccines, cell culture or other fluids.
feature
hydrophilic
manufacturer/tradename
Millistak+®
parameter
1 bar max. differential pressure (15 psid) at 80 °C (Forward)
1 bar max. inlet pressure (15 psi) at 80 °C
2.1 bar max. differential pressure (30 psid) at 25 °C (Forward)
2.1 bar max. differential pressure (30 psid) at 25 °C (Reverse)
3.5 bar max. inlet pressure (50 psi) at 25 °C
H
14 cm (5.3 in.)
L
22 cm (8.5 in.)
filtration area
0.027 m2
impurities
<0.25 EU/mL (LAL test, Aqueous extraction)
input
sample type liquid
conductivity
1.52 – 1.94 μS/cm
filter grade
CE50
shipped in
ambient
Related Categories
General description
This CE50 media series delivers optimal performance through a gradient density matri, ideal for primary (coarse) clarification applications. Millistak+® Pod filters are available in three distinct series of media grades in order to meet your specific needs - DE, CE and HC
.The Millistak+® Pod system is ideal for a wide variety of primary and secondary clarification applications, including cell cultures, yeast and E. coli lysates post centrifuge, E. coli refolds, media, vaccines, plasma proteins and sera.
Easy to Use
With the compact, modular design of our Millistak+® new Pod system, you can increase productivity and shorten cycle times.
Installation and setup of the Pod system is simple and straightforward. The unique design of the disposable adapters and disposable manifolds makes it easy to connect the Pod filters to rest of the unit operations in the process. The self-contained and disposable nature of the system protects operators from exposure to biohazards and eliminates maintenance as well as cleaning validation requirements.
Features and Benefits
- Low hold-up volume for greater product yield
- Broad range of media types offered in single and multilayer products
- Flexible, modular format offers scalability from 5 to 12,000 liters or more
- Patented disposable design eliminates need for housing, CIP or cleaning validation
- Self-contained Pod filters protect operators from exposure to biohazards
- Robust construction is easy to use and set up
- Smaller footprint facilitates use in tight spaces
Specifications
Legal Information
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
A relatively templated manufacturing process can be used to produce live vector vaccines. Challenges exist, however, and include yield loss due to sterile filtration, vector aggregation and scaling adherent cultures. Learn all about this core platform technology and how expertise—empowered by collaboration— can overcome manufacturing challenges.
Manufacturing viral vaccines is complex and there is no templated approach. Each process must be tailored to the shape, size, nature, physico-chemical behavior, stability, and host specificity of the virus. Learn all about this core platform technology and how expertise—empowered by collaboration— can overcome manufacturing challenges.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service