SCCE001
EndoGRO <™Human Umbilical Vein Endothelial Cells (HUVEC)
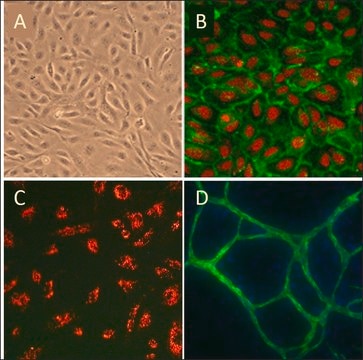
The EndoGRO Human Umbilical Vein Endothelial Cells (HUVEC) are a commonly studied human cell type extracted from human neonatal umbilical cords.
Synonym(s):
HUVEC cells
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41106514
eCl@ss:
32011203
NACRES:
NA.81
Recommended Products
biological source
human umbilical cord vein (endothelium)
Quality Level
packaging
pkg of 500,000 cells
manufacturer/tradename
EndoGRO®
Millipore Chemicon®
morphology
(endothelial)
technique(s)
cell culture | mammalian: suitable
General description
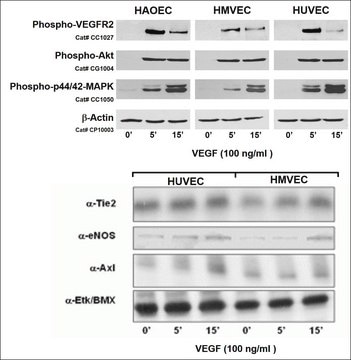
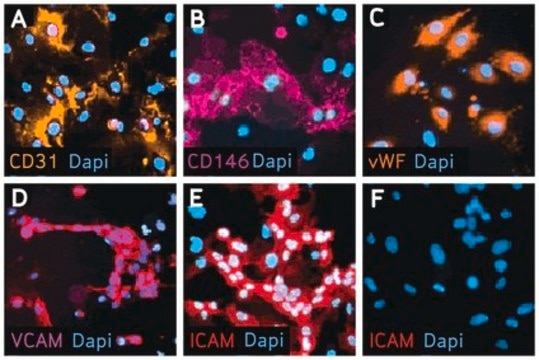
EndoGRO Human Umbilical Vein Endothelial Cells (HUVEC) are a commonly studied human cell type extracted from human neonatal umbilical cords. They are relatively easy to culture, and provide a valuable cell model for many vascular biology research applications, including inflammation, angiogenesis, atherosclerosis, blood clotting, vasoconstriction, and vasodilation. EndoGRO® HUVEC are provided at passage 1, and have been quality tested in low-serum media without exposure to phenol red.
This product is for Research Use Only. This product is not approved for human or veterinary use, or for use in in vitro diagnostic or clinical procedures
This product is for Research Use Only. This product is not approved for human or veterinary use, or for use in in vitro diagnostic or clinical procedures
Product Source: Human Neonatal Umbilical Cords
Cell Line Origin
Umbilical Cord
Cell Line Description
Endothelial Cells
Application
Please see data sheet for detailed directions on Thawing, Plating , Feeding, Passaging, etc.
EndoGRO HUVEC should be cultured in EndoGRO medium (warmed to 37°C) for best results. Please see specific media data sheets for further information (SCME001).
EndoGRO HUVEC should be cultured in EndoGRO medium (warmed to 37°C) for best results. Please see specific media data sheets for further information (SCME001).
Research Category
Stem Cell Research
Stem Cell Research
The EndoGRO Human Umbilical Vein Endothelial Cells (HUVEC) are a commonly studied human cell type extracted from human neonatal umbilical cords.
Packaging
500,000 cells
Components
SCCE001
Quality
Tested negative for Hepatitis B, Hepatitis C and HIV-1. Free of bacteria, fungi and mycoplasma contamination.
Further growth: Guaranteed to provide 15 or more doublings when grown in the appropriate EndoGRO Medium.
Further growth: Guaranteed to provide 15 or more doublings when grown in the appropriate EndoGRO Medium.
Storage and Stability
EndoGRO HUVEC are sold as cryopreserved ampoules. To maintain the cells’ integrity, unpack the products immediately upon receipt. The cryopreserved cells should be placed in the liquid or gas phase of a liquid nitrogen dewar for storage.
Long-term storage of the ampoule must be in a liquid nitrogen dewar or a mechanical freezer designed for cryopreserved cell storage that maintains a temperature below -135°C.
Millipore recommends storing the cryopreserved vials in liquid nitrogen vapor phase. Handle cryopreserved vials with caution. Always wear eye protection and gloves when working with cell cultures. Aseptically vent any nitrogen from cryopreserved vials in a biosafety cabinet prior to thawing the vials in a water bath.
Long-term storage of the ampoule must be in a liquid nitrogen dewar or a mechanical freezer designed for cryopreserved cell storage that maintains a temperature below -135°C.
Millipore recommends storing the cryopreserved vials in liquid nitrogen vapor phase. Handle cryopreserved vials with caution. Always wear eye protection and gloves when working with cell cultures. Aseptically vent any nitrogen from cryopreserved vials in a biosafety cabinet prior to thawing the vials in a water bath.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
ENDOGRO is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service