10976776001
Roche
Nick Translation Kit
sufficient for 50 labeling reactions, kit of 1 (7 components), suitable for FISH
Synonym(s):
nick translation
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41105500
Recommended Products
usage
sufficient for 50 labeling reactions
Quality Level
packaging
kit of 1 (7 components)
manufacturer/tradename
Roche
technique(s)
FISH: suitable
storage temp.
−20°C
General description
Kit for radioactive or nonradioactive labeling of DNA by the nick translation method. This method utilizes a combination of DNase and DNA Polymerase to nick one strand of the DNA helix, then incorporates labeled nucleotides as the polymerase examines, or "proofreads" the nicked site.
Specificity
Heat inactivation: Stop the reaction by adding 1μl 0.5 M EDTA (pH 8.0) and/or by heating to 65 °C for 10 minutes.
Application
Nick translation kit has been used in FISH (fluorescence in situ hybridization) based labeling of tick and bacterial artificial chromosome. It has been used in labeling the probe (genomic DNA) in order to obtain GISH (genomic in situ hybridization) signals.
The kit is used for labeling of DNA with radioactive or modified dNTPs. Probes labeled by nick translation are used in many different hybridization techniques. These include use as probes in:
- Screening gene banks by colony- or plaque hybridization
- DNA or RNA transfer hybridizations
- In situ hybridization
- Reassociation kinetic studies
Packaging
1 kit containing 7 components.
Specifications
Assay Time: 45minutes
Specific Activity: The level of specific labeling and the incorporation rate are dependent on the ratio of substrate DNA to labeled deoxyribonucleoside triphosphate, e.g., the kinetics and labeling levels obtained are identical in assays containing 0.1μg DNA and 20μCi dXTP or 0.5μg DNA and 100μCi dXTP.
The standard assay will give a specific activity of 3 x 108 dpm/μg, corresponding to 65% incorporation with different substrate DNAs (e.g., pBR322, λDNA, DNA fragments) in 35minutes.
Sample Materials
Specific Activity: The level of specific labeling and the incorporation rate are dependent on the ratio of substrate DNA to labeled deoxyribonucleoside triphosphate, e.g., the kinetics and labeling levels obtained are identical in assays containing 0.1μg DNA and 20μCi dXTP or 0.5μg DNA and 100μCi dXTP.
The standard assay will give a specific activity of 3 x 108 dpm/μg, corresponding to 65% incorporation with different substrate DNAs (e.g., pBR322, λDNA, DNA fragments) in 35minutes.
Sample Materials
- Supercoiled and linearized plasmid DNA
- Supercoiled and linearized cosmid DNA
- BAC DNA or human genomic DNA
- Purified PCR products
Principle
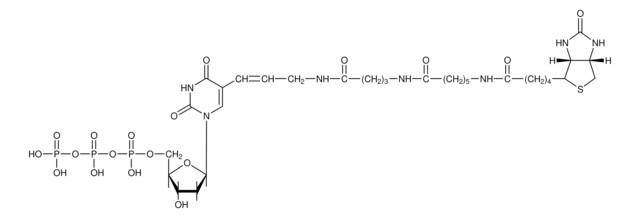
The nick translation method is based on the ability of DNase I to introduce randomly distributed nicks into DNA at low enzyme concentrations in the presence of Mg2+. E. coli DNA polymerase I synthesizes DNA complementary to the intact strand in a 5′→3′ direction using the 3′-OH termini of the nick as a primer. The 5′→3′ exonucleolytic activity of DNA polymerase I simultaneously removes nucleotides in the direction of synthesis. The polymerase activity sequentially replaces the removed nucleotides with isotope-labeled or hapten-labeled deoxyribonucleoside triphosphates. At low temperature (+15°C), the unlabeled DNA in the reaction is thus replaced by newly synthesized labeled DNA.
Preparation Note
Working concentration: The recommended working concentration with T7, T3 or SP6 RNA Polymerases is 1 mM.
Working solution: DNA
The DNA must be in low-salt concentration solution.
dATP, dGTP, dTTP
Working solution: DNA
The DNA must be in low-salt concentration solution.
dATP, dGTP, dTTP
- If the same labeled deoxyribonucleoside triphosphate is used repeatedly, we recommend the preparation of a mixture of equal parts of the other three triphosphates for convenience.
- Prepare the dATP, dGTP, dTTP mixture by making a 1:1:1 mixture of solution 2, solution 4, and solution 5.
Other Notes
For life science research only. Not for use in diagnostic procedures.
Kit Components Only
Product No.
Description
- Control DNA pBR322, 20 μl 50 µg/ml
- dATP, 50 μl 0.4 mM
- dCTP, 50 μl 0.4 mM
- dGTP, 50 μl 0.4 mM
- dTTP, 50 μl 0.4 mM
- Nick Translation Buffer, 100 μl 10x concentrated
- Enzyme Mixture, 100 μl, DNA Polymerase I and DNase I in 50% glycerol (v/v)
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
does not flash
flash_point_c
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amy Sebeson et al.
PloS one, 10(5), e0127214-e0127214 (2015-05-21)
The binding sequence for any transcription factor can be found millions of times within a genome, yet only a small fraction of these sequences encode functional transcription factor binding sites. One of the reasons for this dichotomy is that many
A methylation status analysis of the apomixis-specific region in Paspalum spp. suggests an epigenetic control of parthenogenesis.
Podio M, et al.
Journal of Experimental Botany, 65(22), 6411-6424 (2014)
David U Gorkin et al.
Genome biology, 20(1), 255-255 (2019-11-30)
The 3-dimensional (3D) conformation of chromatin inside the nucleus is integral to a variety of nuclear processes including transcriptional regulation, DNA replication, and DNA damage repair. Aberrations in 3D chromatin conformation have been implicated in developmental abnormalities and cancer. Despite
Aiko Iwata-Otsubo et al.
Current biology : CB, 27(15), 2365-2373 (2017-08-02)
Female meiosis provides an opportunity for selfish genetic elements to violate Mendel's law of segregation by increasing the chance of segregating to the egg [1]. Centromeres and other repetitive sequences can drive in meiosis by cheating the segregation process [2]
Anna Zaleśna et al.
Comparative cytogenetics, 11(2), 249-266 (2017-09-19)
We studied water frogs from a complex composed of two species: Pelophylax lessonae (Camerano, 1882) (genome LL, 2n = 26) and P. ridibundus (Pallas, 1771) (RR, 2 = 26), and their natural hybrid P. esculentus (Fitzinger, 1843) of various ploidy
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service