COLLH-RO
Roche
Collagenase H
from Clostridium histolyticum
About This Item
Recommended Products
biological source
Clostridium histolyticum
Quality Level
sterility
non-sterile
form
lyophilized
collagenase activity
0.15 U/mg lyophilizate
packaging
pkg of 100 mg (11074032001)
pkg of 2.5 g (11087789001)
pkg of 500 mg (11074059001)
manufacturer/tradename
Roche
concentration
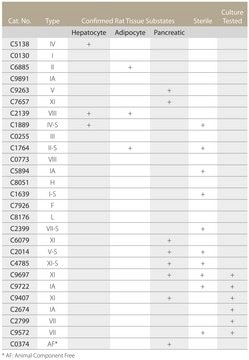
~1 mg/mL (Isolation of rat hepatocytes)
~2 mg/mL (Isolation of adipocytes)
technique(s)
tissue processing: suitable
color
brown
optimum pH
6.0-8.0
solubility
water: soluble
NCBI accession no.
UniProt accession no.
application(s)
life science and biopharma
foreign activity
Clostripain 39.798 U/mg
Proteases 14.014 U/mg (Azocoll, based on lyoph)
Trypsin 1.668 U/mg (with BAEE)
storage temp.
2-8°C
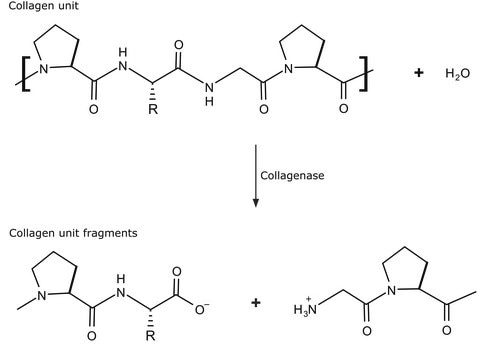
General description
Specificity
Application
Physical form
Preparation Note
Collagenase activity: >0.15U/mg (according to Wünsch) (+25°C, 4-phenyl-azobenzyl-oxycarbonyl-Pro-Leu-Gly-Pro-D-Arg as the substrate).
Contaminating enzyme activities: trypsin, clostripain, and total proteolytic activity
Collagenase H has a balanced ratio of enzyme activities, and is function tested for the isolation of rat hepatocytes (perfusion method).
Inhibitors:
Collagenase inhibitors: EDTA, EGTA, Cys, His, DTT, 2-mercaptoethanol
Collagenase is not inhibited by serum.
Clostripain inhibitors: TLCK
Trypsin inhibitors: aprotinin, trypsin inhibitor (egg white, soybean)
Activator: Ca2+
Working concentration: 0.5 to 2.5mg/ml,
Approximately 1 mg/ml for the isolation of rat hepatocytes and 2mg/ml for the isolation of adipocytes.
Storage conditions (working solution): -15 to -25°C
The reconstituted solution should be stored at -15 to -25°C for short term storage, -60°C or as described below for long-term storage.
Reconstitution
Storage and Stability
Other Notes
signalword
Danger
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
does not flash
flash_point_c
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Enzyme Explorer Key Resource: Collagenase Guide.Collagenases, enzymes that break down the native collagen that holds animal tissues together, are made by a variety of microorganisms and by many different animal cells.
Related Content
Collagenase Guide.Collagenases, enzymes that break down the native collagen that holds animal tissues together, are made by a variety of microorganisms and by many different animal cells.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service