All Photos(3)
About This Item
Empirical Formula (Hill Notation):
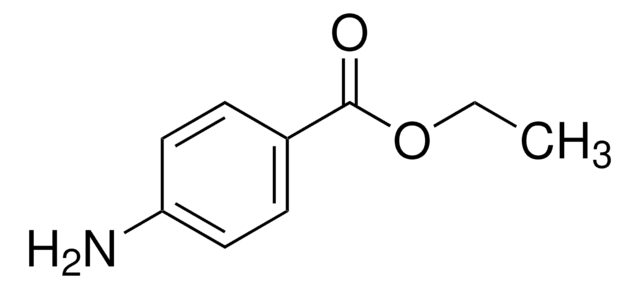
C11H15NO2
CAS Number:
Molecular Weight:
193.24
Beilstein/REAXYS Number:
2803178
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98.0% (NT)
reaction suitability
reaction type: solution phase peptide synthesis
color
white to almost white
mp
108-110 °C
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)c1ccc(N)cc1
InChI
1S/C11H15NO2/c1-11(2,3)14-10(13)8-4-6-9(12)7-5-8/h4-7H,12H2,1-3H3
InChI key
KYORUZMJUKHKFS-UHFFFAOYSA-N
Other Notes
Building block for the synthesis of antifolates, important as tumor cell inhibitors
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L F Hennequin et al.
Journal of medicinal chemistry, 39(3), 695-704 (1996-02-02)
Modification of the potent thymidylate synthase (TS) inhibitor 1-[[N-[4-[N-[(3,4-dihydro-2-methyl-4-oxo-6-quinazolinyl)methyl]-N- prop-2-ynylamino]benzoyl]amino]methyl]-3-nitrobenzene (4a) has led to the synthesis of quinazolinone antifolates bearing functionalized alkyl substituents at C2. A general synthetic route was developed which involved coupling the appropriate 1-[[N-[4-(alkylamino)benzoyl)amino]methyl]-3-nitrobenzene 20-22 with a
G.M.F. Visset et al.
Journal of Medicinal Chemistry, 35, 659-659 (1992)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service