09236
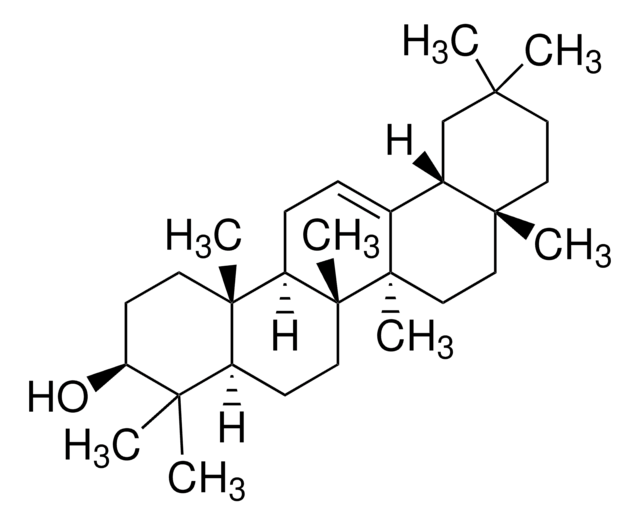
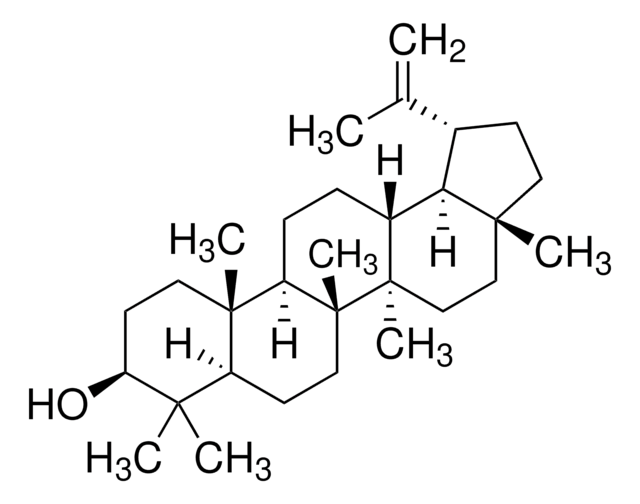
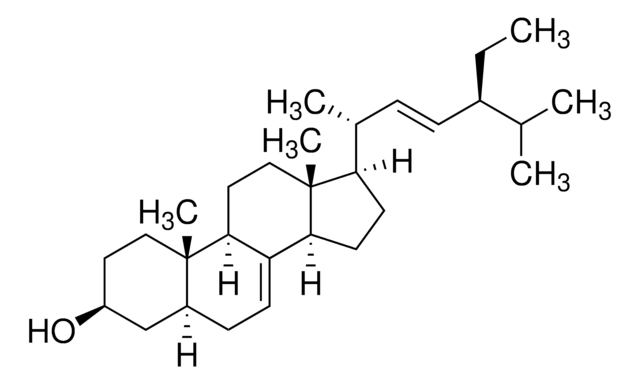
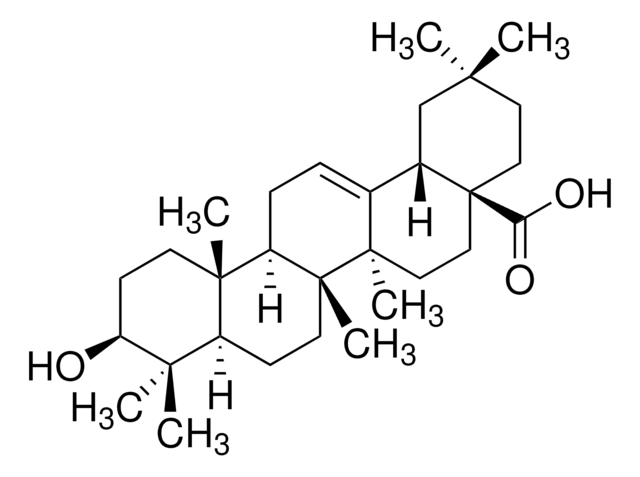
β-Amyrin
analytical standard
Synonym(s):
β-Amyrenol, Olean-12-en-3β-ol
About This Item
Recommended Products
grade
analytical standard
Quality Level
assay
≥98.5% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
food and beverages
format
neat
SMILES string
[H][C@@]12CC(C)(C)CC[C@]1(C)CC[C@]3(C)C2=CC[C@]4([H])[C@@]5(C)CC[C@H](O)C(C)(C)[C@]5([H])CC[C@@]34C
InChI
1S/C30H50O/c1-25(2)15-16-27(5)17-18-29(7)20(21(27)19-25)9-10-23-28(6)13-12-24(31)26(3,4)22(28)11-14-30(23,29)8/h9,21-24,31H,10-19H2,1-8H3/t21-,22-,23+,24-,27+,28-,29+,30+/m0/s1
InChI key
JFSHUTJDVKUMTJ-QHPUVITPSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Development and validation of two high-performance thin layer chromatography (HPTLC) methods to quantify four biomarkers— β-amyrin, β-sitosterol, lupeol, and ursolic acid in the ethanol extract of G. senegalensis leaves
- Analysis of methanol extracts of leaf samples collected from five Ficus species by a novel HPTLC densitometric method, for the determination of β-amyrin, validated according to the International Conference on Harmonization (ICH) guidelines
- Quantification of β-amyrin in the ethanolic extracts collected from two Maytenus species, M. obscura and M. parviflora by a validated HPTLC-densitometric method
- Secondary metabolite profiling of various plant parts collected from 82 plants belonging to 21 different cannabis strains using gas chromatography-mass spectrometry (GC-MS) for sterols and terpenoids (mono-, sesqui-, tri-), and high-performance liquid chromatography (HPLC) with UV and mass spectrometric (MS) detection for flavonoids
Biochem/physiol Actions
Packaging
signalword
Warning
hcodes
pcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service