About This Item
Recommended Products
Quality Level
assay
≥99.0%
99.0-101.0% (dried substance)
form
powder or crystals
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
DNA synthesis | interferes
enzyme | inhibits
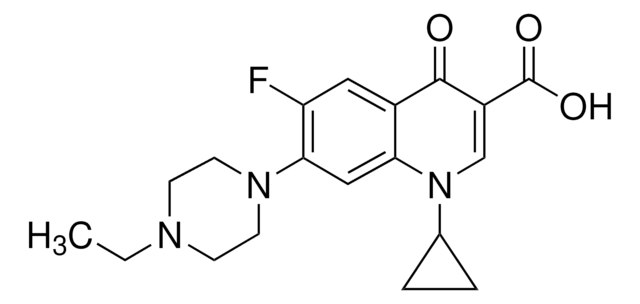
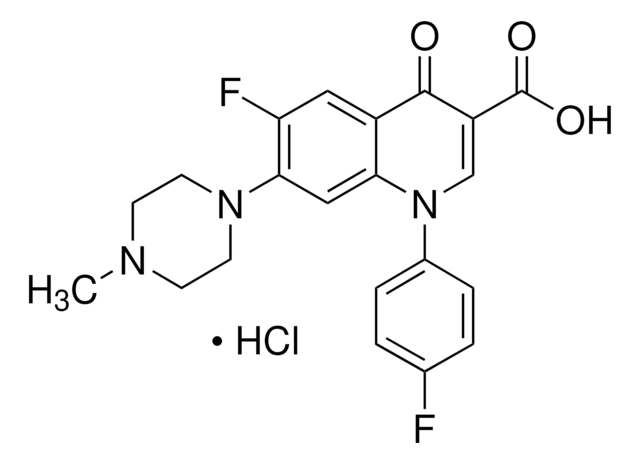
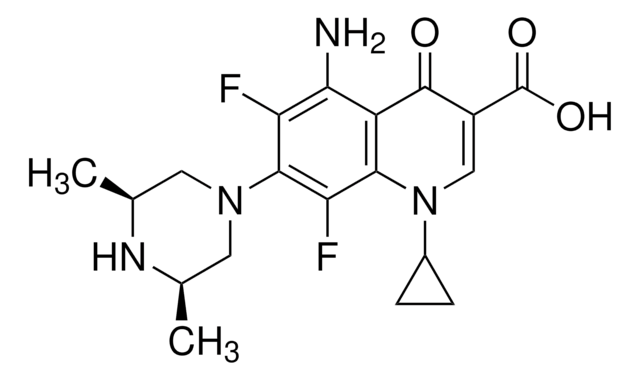
SMILES string
CCN1CCN(CC1)c2cc3N(C=C(C(O)=O)C(=O)c3cc2F)C4CC4
InChI
1S/C19H22FN3O3/c1-2-21-5-7-22(8-6-21)17-10-16-13(9-15(17)20)18(24)14(19(25)26)11-23(16)12-3-4-12/h9-12H,2-8H2,1H3,(H,25,26)
InChI key
SPFYMRJSYKOXGV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- to investigate the pathological mechanisms resulting from the toxicity of fluoroquinolones in mammalian cells
- to prepare a standard solution with 50% acetonitrile in a study to analyze illegal fish drugs used in aquaculture by employing surface-enhanced Raman spectroscopy (SERS)
- as an analyte in a chemiluminescence reagent system
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Resp. Sens. 1 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service